The Spectrum of Patients* With Type 2 Inflammation in Asthma present with Various Disease Characteristics

IgE=immunoglobulin E
*Hypothetical patient profiles.
.jpg/jcr:content/1.2022-08-22-11-00-55%20(2).jpg)
Gene expression measures in airway epithelial brushings from asthmatic patients with mild-to-moderate asthma.1
50% To 70% Of adult patients with asthma have type 2 inflammation adapted from ref. 1
Gina guidelines for difficult-to-treat and severe asthma
The possibility of Refractory Type 2 Inflammation should be considered if any of the following are found while the patient is taking high dose ICS or daily OCS:2
- Blood eosinophils ≥150 cells/μL
- Sputum eosinophils ≥2%
- Asthma is clinically allergen-driven
- FeNO ≥20 ppb
Biomarkers of Type 2 inflammation may be suppressed by systemic corticosteroid use. This should be taken into consideration when determining Type 2 status in patients taking oral corticosteroids3
GINA= Global Initiative for Asthma; ICS= inhaled corticosteroid; FeNO= fractional exhaled nitric oxide; OCS= oral corticosteroid.
References
- Peters MC, Mekonnen BA, Yuan S, et al. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol. 2014; 133 (2): 388–394
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2020 update). Available at: https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf. Last accessed: 8/3/2021
- DUPIXENT Summary of Product Characteristics. September 2017. Available at: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf. Last accessed at: 13.9.2021.
Type 2 cytokines drive the recruitment of effector cells (e.g. eosinophils) and mediate class switching of B cells to secrete IgE upon exposure to antigens. They are also central to many of the hallmarks of asthma.1
IL-4, which acts as a regulatory cytokine upstream from type 2 effector cytokines, IL-13 has been described as a key effector cytokine due to the multifunctional role that it plays in many aspects of asthma pathogenesis.1
.jpg/jcr:content/24%20(1).jpg)
Gina (2020) guideline decision tree2
.jpg/jcr:content/25%20(1).jpg)
+, ++, +++: Plus signs indicate the strength of an association
FeNO= fractional exhaled nitric oxide; GINA= Global Initiative for Asthma; ICS= inhaled corticosteroid; IgE= immunoglobulin E; IL= interleukin; IV= intravenous; LABA=long-acting β-agonist; OCS= oral corticosteroid; SC= subcutaneous; IL5R= Interleukin-5 receptor.
References
- Robinson D, Humbert , Buhl R, et al. Revisiting type 2-high and type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017; 47 (2): 161-175.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2020 update). Available at: https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf. Last accessed: 8/3/2021.
Type 2 asthma includes a range of phenotypes1
.jpg/jcr:content/38%20(1).jpg)
GINA recommends adding on a Type 2 biologic for patients with exacerbations on high-dose ICS/LABA, with or without daily OCS2
GINA=Global Initiative for Asthma; ICS=inhaled corticosteroid; LABA=long-acting β-agonist; OCS=oral corticosteroid.
References
- Tran TN, Zeiger RS, Peters SP, et al. Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol. 2016;116 (1): 37-42.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2020 update). Available at: https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf. Last accessed: 8/3/2021
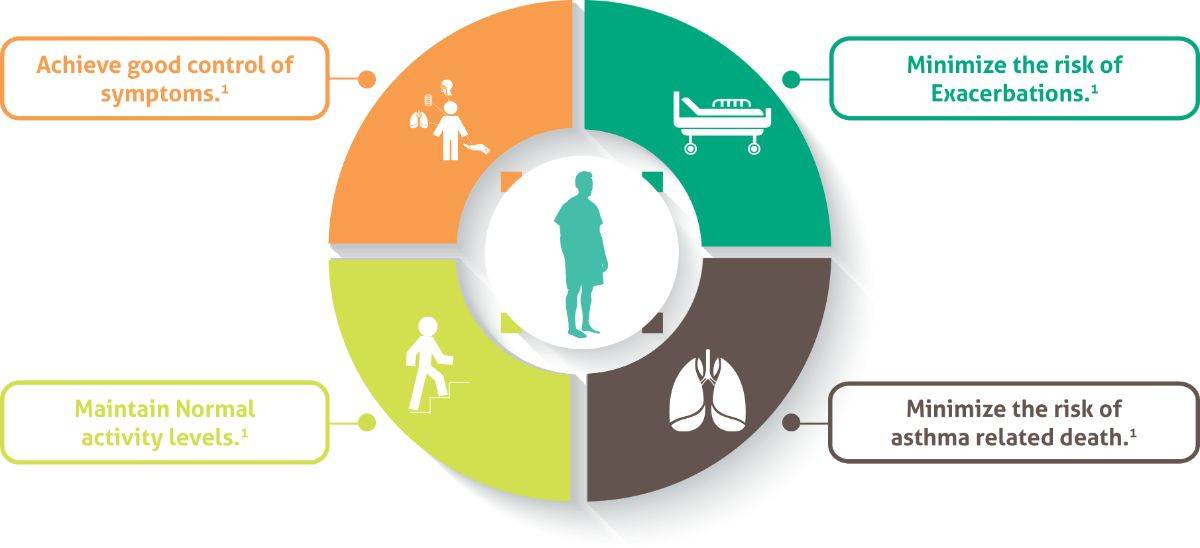
Long-term Goals of Asthma Management1
Poor asthma control is associated with increased risk of exacerbations, debilitation, impaired quality of life, increased health-care utilisation and reduced productivity.2
.jpg)
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention (2020 update). Available at: https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf. Last accessed: 8/3/202.
- Price D, et al. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med.2014; 24 : 14009
Dupixent provides dual inhibition of Interleukin-4 and Interleukin-13
Adapted from ref. 1
Dupilumab is a recombinant human monoclonal antibody that inhibits interleukin-4 and interleukin-13 signaling. Dupilumab inhibits IL-4 signaling via the Type I receptor (IL-4Rα/γc). and both IL-4 and IL-13 signaling through the Type II receptor (IL-4Rα/IL -13Rα). IL-4 and IL-13 are major drivers of human type 2 inflammatory disease, such as atopic dermatitis and asthma. Blocking the IL-4/IL- 13 pathway with dupilumab in patients decreases many of the mediators or Type 2 Inflammation.1
IL-4= interleukin-4; IL-13= interleukin-13
Reference
- Dupixent Summary of Product Characteristics. September 2017. Available at: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf. Last accessed at: 13.9.2021.
Liberty asthma quest.1*
This is a randomized, double-blind, placebo-controlled, parallel-group, phase trial.1
.jpg/jcr:content/27%20(1).jpg)
Primary Endpoint1
- Annualised rate of severe exacerbation† events over 52 weeks
- Change from baseline to Week 12 in FEV1 (L)
Key Secondary Endpoints1
- Primary endpoints assessed in subgroups based on baseline blood EOS and FeNO
- Asthma control and quality-of-life scores
EOS=eosinophil; FeNO=fractional exhaled nitric oxide; FEV1=forced expiratory volume in 1 second; L=litres; Q2W=every 2 weeks; SOC=standard of care.
* Patients were recruited irrespective of a minimum baseline blood eosinophil count or biomarkers of type 2 inflammation.1
Asthma treatment guidelines define Type 2 inflammation as eosinophilia ≥150 cells/μL and/or FeNO ≥20 ppb.2 †Deterioration of asthma leading to treatment for ≥3 days with systemic CS or hospitalisation/emergency department visit leading to treatment with systemic CS.1
The dupilumab 200-mg group was initially given a 400-mg loading dose. The dupilumab 300-mg group was initially given a 600-mg loading dose.1
References
- Castro M, Corren J, Pavord ID, Maspero J, Wenze1l S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. New England Journal of Medicine. 2018;378 (26): 2486- 96.
- DUPIXENT Summary of Product Characteristics. September 2017. Available at: https://www.ema.europa.eu/en/documen2ts†/product-information/dupixent-epar-product-information_en.pdf. Last accessed at: 13.9.2021.
Dupixent was studied in uncontrolled moderate to severe asthma patients
Adapted from ref. 1
Patients were enrolled regardless of minimum eosinophil count or other Type 2 biomarkers1
Across Two Trials
Atopic Comorbidities at Baseline2
77% of Patients Reported atopic medical history
- 10% had atopic dermatitis
- 16% had nasal polyposis
- 65% had allergic rhinitis
Additional baseline characteristics1
.png)
Mean age: 42 Years
.png)
High-Dose ICS Use: 54.3 %
.png)
Mean Exacerbations in Previous Year 2.07
References
- Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. New England Journal of Medicine. 2018; 378(26): 2486-96.
- Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting ß2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. The Lancet. 2016; 388 (10039): 31-44.
Patients who received dupilumab had greater reductions from baseline in the levels of total immunoglobulin e.1
Liberty Asthma Quest trial: patients aged ≥12 years1
.jpg)
.jpg)
IgE=immunoglobulin E; LABA=long-acting β-agonist; LAMA=long-acting muscarinic antagonist; LD=loading dose; LTRA=leukotriene receptor antagonist; SABA=short-acting β-agonist; SOC=standard of care.
*Calculation based on reported group means at baseline and Week 52.
Study participants were randomised 2:2:1:1 to receive dupilumab 200 mg (LD=400 mg) or 300 mg (LD=600 mg) as add-on therapy every 2 weeks or a matched-volume placebo (1.14 mL or 2.00 mL, respectively) for each active dose. SOC therapy permitted throughout study included LABA, LAMA, LTRA, methylxanthines, and SABA (as needed).1
References
- Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. New England Journal of Medicine. 2018; 378(26): 2486-96.
- Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting ß2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. The Lancet. 2016; 388 (10039): 31-44.
Dupilumab efficacy in uncontrolled, moderate-to-severe allergic asthma: adjusted annualized severe exacerbation rate by allergic status1
Allergic asthma was defined as total serum IgE ≥ 30 kU/L and ≥ 1 positive perennial aeroallergen-specific IgE (≥ 0.35 kU/L) at baseline1

Met the allergic asthma criteria1

Did not meet the allergic asthma criteria1

Placebo 200 mg q2w

Dupilumab 200 mg q2w

Placebo 300 mg q2w

Dupilumab 300 mg q2w
Dupilumab significantly reduced severe exacerbations in patients who met or did not meet criteria for allergic asthma1
CI=confidence interval; q2w=every 2 weeks.
**p<0.01; ***p<0.001 versus placebo.
Reference
- Corren J, Castro M, O'Riordan T et al. Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Allergic Asthma. J Allergy Clin Immunol Pract. 2020 ; 8 (2): 516-526.
The rate of the most severe asthma exacerbations, those leading to hospitalization or emergency department visits, was also significantly lower with dupilumab than with placebo.1*
.png/jcr:content/78.webp)
- A severe exacerbation event was defined as a deterioration of asthma requiring use of systemic corticosteroids for ≥3 days or hospitalization or emergency department visit1
Recalling the symptoms during the previous week and to respond to the first 6 questions2
- Night time waking
- Symptoms upon waking
- Activity limitations
- Shortness of breath
- Wheezing
SOC= Standard of care.
* P<0.001
References
- Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018; 378 (26): 2486-2496.
- Meltzer EO, Busse WW, Wenzel SE, et al. Use of the Asthma Control Questionnaire to predict future risk of asthma exacerbation. J Allergy Clin Immunol. 2011; 127 (1): 167-172.
Dupixent dosage and administration.1
Dupilumab is recommended for patients aged ≥12 years in 2 dosing regimens1
.jpg/jcr:content/20%20(1).jpg)
Dupilumab 300 mg for
OCS-dependent severe asthma or severe asthma with comorbid atopic dermatitis*1
.jpg/jcr:content/22%20(1).jpg)
Dupilumab 200 mg for
All other indicated patients1
- Dupixent is used with other asthma medicines for the maintenance of severe asthma in adults and adolescents ( 12 years and older) whose asthma is not controlled with their current asthma medicines.1
- Dupixent help to reduce the use of oral corticosteroids.1
If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing at the regular scheduled time.2
Administration considerations1
- Dupilumab is administered by subcutaneous injection into the thigh or abdomen
- Dupilumab should not be injected into skin that is tender, damaged, or has bruises or scars
- Except for the 5 cm around the navel (if somebody else administers the injection, the upper arm can also be used).1
OCS= oral corticosteroid.
*Moderate-to-severe atopic dermatitis
References
- Egyptian drug authority Dupixent approval leaflet date 6/1/2021.
- Dupixent Summary of Product Characteristics. October 2019. Available at: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf. Last accessed at: 13.9.2021
Dupixent overview of adverse events during 52-week intervention period1
Adverse Events That Emerged during the Intervention Period (Safety Population).*1 “Quest trial”
|
Event |
Placebo, 1.14 ml (N=313) |
Dupilumab, 200 mg (N=631) |
Placebo, 2.00 ml (N=321) |
Dupilumab, 300 mg (N=632) |
Combined Placebo (N=634) |
Combined Placebo (N=1263) |
| Any adverse event |
257 (82.1) |
508 (80.5) |
270 (84.1) |
515 (81.5) |
527 (83.1) |
1023 (81.0) |
| Any serious adverse event |
26 (8.3) |
49 (7.8) |
27 (8.4) |
55 (8.7) |
53 (8.4) |
104 (8.2) |
| Any adverse event leading to death† |
3 (1.0) |
1 (0.2) |
0 |
4 (0.6) |
3 (0.5) |
5 (0.4) |
| Any adverse event leading to permanent discontinuation of the invervention |
19 (6.1) |
19 (3.0) |
10 (3.1) |
44 (7.0) |
29 (4.6) |
63 (5.0) |
| Adverse events occurring in ≥5% of patientsin any group† | ||||||
| Headche |
26 (8.3) |
46 (7.3) |
25 (7.8) |
40 (6.3) |
51 (8.0) |
86 (6.8) |
| Injection-site reaction¶ |
17 (5.4) |
96 (15.2) |
33 (10.3) |
116 (18.4) |
50 (7.9) |
212 (16.8) |
*The safety population included all the patients who received at least one dose or part of a dose, and data were analyzed according to the intervention received. Patients received dupilumab at a dose of 200 or 300 mg every 2 weeks or a matched-volume placebo.
†Causes of death in the dupilumab groups were pulmonary embolism, cardiopulmonary arrest in a patient with paraplegia due to spinal cord injury and multiple vertebral fractures due to osteoporosis, respiratory depression with cardiorespiratory arrest and ischemic encephalopathy, unwitnessed death attributed to myocardial infarction, and cardiac congestive failure with ventricular tachycardia in an obese patient with a history of obstructive sleep apnea. In the placebo groups, deaths were attributed to recurrence of thyroid cancer,postoperative pulmonary embolism after knee arthroplasty, and suicide.
‡Adverse events in this category were reported according to the preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA), version 20.0, unless otherwise indicated.
¶Injection-site reaction is a high-level term in MedDRA.
MedDRA= Medical Dictionary for Regulatory Activities.
Reference
- Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. New England Journal of Medicine. 2018;378 (26): 2486-96.
Dupixent inhibits IL-4 and IL-13 signaling, which are the mediators of Type 2 inflammation.1
- 47.7% Lower rate with Dupilumab than with Placebo.
The adjusted annualized rate of severe asthma [CI], 0.39 to 0.53) among patients assigned to 200 mg of dupilumab every 2 weeks versus 0.87 (95% CI, 0.72 to 1.05) among those assigned to matched placebo (P<0.001).2
- No loss with either Dupilumab dose.
Prespecified analysis of the rate of change in the postbronchodilator FEV1 (FEV1 slope after week 4 to week 52) showed a loss of lung function of 40 ml per year with placebo.2
IL-4= interleukin-4; IL-13= interleukin-13; FEV1= forced expiratory volume in 1 second.
References
- Dupixent Summary of Product Characteristics. September 2017. Available at: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf. Last accessed at: 13.9.2021.
- Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018; 378 (26): 2486-2496.
Package Leaflet: Information for the User
Dupixent® 200mg
solution for injection in pre-filled syringe Dupilumab
What is the Drug
Dupixent contains the active substance Dupilumab. Dupilumab is a monoclonal antibody (a type of specialized protein) that blocks the action of proteins called IL-4 and IL-13. Both play a major role in causing the signs and symptoms of Asthma.
Indication and Usage
Dupixent is used to treat adults and adolescents 12 years and older with moderate-to-severe Atopic dermatitis, also known as Atopic eczema. Dupixent may be used with Eczema medicines that you apply to the skin or it may be used on its own.
Using Dupixent for Atopic dermatitis (Atopic eczema) can improve the condition of your skin and reduce itching. Dupixent has also been shown to improve symptoms of pain, Anxiety, and Depression associated with Atopic dermatitis. In addition, Dupixent helps improve your sleep disturbance and overall quality of life. Dupixent is used with other Asthma medicines for the maintenance treatment of Severe asthma in adults and adolescents (12 years of age and older) whose Asthma is not controlled with their current Asthma medicines.
Dupixent helps prevent severe asthma attacks (exacerbations) and can improve your breathing.
Dupixent may also help reduce the amount of another group of medicines you need to control your Asthma, called Oral corticosteroids, while preventing Severe asthma attacks and improving your breathing
Always use this medicine exactly as your Doctor or Pharmacist has told you. Check with your Doctor or Pharmacist if you are not sure
Dosage and Administration
Your Doctor will decide how much Dupixent you need and for how long. Dupixent is given by injection under the skin (subcutaneous injection). Recommended dose in adolescents with Atopic dermatitis The recommended dose of Dupixent for adolescents (12 to 17 years of age) with Atopic dermatitis is based on body weight:
| Body Weight of Patient | Initial dose | Subsequent Doses (every other week) |
| Less than 60kg | 400mg (two 200mg injections) | 200mg |
| 60kg or more | 600mg (two 300mg injections) | 300mg |
Recommended dose in adult and adolescent patients with Asthma For Asthma, the recommended dose of Dupixent for adult and adolescents patients (12 years of age and older) is:
- An initial dose of 400 mg (two 200 mg injections), followed by 200 mg given every other week administered as subcutaneous injection.
- For patients with Severe asthma and who are on Oral corticosteroids or for patients with Severe asthma and comorbid moderate-to-severe Atopic dermatitis, an initial dose of 600 mg (two 300 mg injections), followed by 300 mg every other week administered as subcutaneous injection.
Dupixent is given by injection under your skin (subcutaneous injection). You and your Doctor or nurse should decide if you should inject Dupixent yourself. Inject Dupixent yourself only after you have been trained by your Doctor or Nurse. A Caregiver may also give you your Dupixent injection after proper training. Each pre-filled syringe contains one dose of Dupixent (200 mg). Do not shake the pre-filled syringe
Contraindications
Do not use Dupixent
- If you are allergic to Dupilumab or any of the other ingredients of this medicine (listed in section 6).
- If you think you may be allergic, or you are not sure, ask your Doctor, Pharmacist or nurse for advice before using Dupixent
Warning and Precautions
Talk to your Doctor, Pharmacist or nurse before using Dupixent:
Dupixent is not a rescue medicine and should not be used to treat a sudden Asthma attack. Allergic reactions Very rarely, Dupixent can cause serious side effects, including allergic (hypersensitivity) reactions and anaphylactic reaction. You must look out for signs of these conditions (i.e. breathing problems, swelling of the face, mouth, and tongue, fainting, dizziness, feeling lightheaded (low blood pressure), fever, general ill feeling, swollen lymph nodes, hives, itching, joint pain, skin rash) while you are taking Dupixent. Stop using Dupixent and tell your Doctor or get medical help immediately if you notice any signs of an allergic reaction. Such signs are listed under “Serious side effects” in section 4.
Eosinophilic conditions Rarely patients taking an Asthma medicine may develop inflammation of blood vessels or lungs due to an increase of certain white blood cells (Eosinophilia). It is not known whether this is caused by Dupixent. This usually, but not always, happens in people who also take a steroid medicine which is being stopped or for which the dose is being lowered. Tell your doctor immediately if you develop a combination of symptoms such as a flu-like illness, pins and needles or numbness of arms or legs, worsening of Pulmonary symptoms, and/or rash. Parasitic (Intestinal parasites) infection Dupixent may weaken your resistance to infections caused by parasites. If you already have a Parasitic infection it should be treated before you start treatment with Dupixent. Check with your doctor if you have Diarrhea, gas, upset stomach, greasy stools, and Dehydration which could be a sign of a Parasitic infection. If you live in a region where these infections are common or if you are travelling to such a region check with your doctor.
Asthma If you have Asthma and are taking Asthma medicines, do not change or stop your Asthma medicine without talking to your Doctor. Talk to your Doctor before you stop Dupixent or if your Asthma remains uncontrolled or worsens during treatment with this medicine. Eye problems (if you have Atopic dermatitis) Talk to your Doctor if you have any new or worsening eye problems, including eye pain or changes in vision.
Children and Adolescents
The safety and benefits of Dupixent are not yet known in children and adolescents with Atopic dermatitis below the age of 12. The safety and benefits of Dupixent are not yet known in children with Asthma below the age of 12.
Adverse reaction
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Dupixent can cause serious side effects, including very rare allergic (hypersensitivity) reactions, including anaphylactic reaction; the signs of allergic reaction or anaphylactic reaction may include:
breathing problems, swelling of the face, mouth, and tongue, fainting, dizziness, feeling lightheaded (low blood pressure), fever, general ill feeling, swollen lymph nodes, hives, itching, joint pain skin rash, If you develop an allergic reaction, stop using Dupixent and talk to your Doctor right away.
Other side effects
Very Common (may affect more than 1 in 10 people) Atopic dermatitis and Asthma:
injection site reactions (i.e. redness, swelling, and itching)
Common (may affect up to 1 in 10 people) atopic dermatitis only:
Headache, eye dryness, redness and itching, eyelid itching, redness and swelling eye infection, cold sores (on lips and skin)
Drug interactions
Tell your Doctor or Pharmacist
- If you are using, have recently used or might use any other medicines.
- If you have recently had or are due to have a vaccination.
Do not stop or reduce your Asthma medicines, unless instructed by your Doctor. These medicines (especially ones called Corticosteroids) must be stopped gradually, under the direct supervision of your Doctor and dependent on your response to Dupixent.
Pregnancy and breast-feeding
If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your Doctor for advice before using this medicine. The effects of this medicine in pregnant women are not known; therefore it is preferable to avoid the use of Dupixent in pregnancy unless your Doctor advises to use it.
If you are breast-feeding or are planning to breast-feed, talk to your Doctor before using this medicine. You and your Doctor should decide if you will breastfeed or use Dupixent. You should not do both.
Driving and using machines
Dupixent is unlikely to influence your ability to drive and use machines.
- Always read the full prescribing information.
- Healthcare professionals are asked to report any suspected adverse reactions to the Egyptian Pharmacovigilance Center (EPVC).
- Egyptian drug authority dupixent leaflet approval date 6/1/2021.
Package Leaflet: Information for the User
Dupixent® 300mg
solution for injection in pre-filled syringe Dupilumab
What is the Drug:
Dupixent contains the active substance Dupilumab. Dupilumab is a monoclonal antibody (a type of specialized protein) that blocks the action of proteins called IL-4 and IL-13. Both play a major role in causing the signs and symptoms of Asthma.
Indication and Usage:
Dupixent is used to treat adults and adolescents 12 years and older with moderate-to-severe Atopic dermatitis, also known as Atopic eczema. Dupixent may be used with Eczema medicines that you apply to the skin or it may be used on its own.
Using Dupixent for Atopic dermatitis (Atopic eczema) can improve the condition of your skin and reduce itching. Dupixent has also been shown to improve symptoms of pain, anxiety, and depression associated with Atopic dermatitis. In addition, Dupixent helps improve your sleep disturbance and overall quality of life. Dupixent is used with other Asthma medicines for the maintenance treatment of Severe asthma in adults and adolescents (12 years of age and older) whose Asthma is not controlled with their current Asthma medicines.
Dupixent helps prevent Severe asthma attacks (exacerbations) and can improve your breathing.
Dupixent may also help reduce the amount of another group of medicines you need to control your Asthma, called Oral corticosteroids, while preventing Severe asthma attacks and improving your breathing
Always use this medicine exactly as your Doctor or Pharmacist has told you. Check with your Doctor or Pharmacist if you are not sure
Dosage and Administration:
Your Doctor will decide how much Dupixent you need and for how long. Dupixent is given by injection under the skin (subcutaneous injection). Recommended dose in adolescents with Atopic dermatitis The recommended dose of Dupixent for adolescents (12 to 17 years of age) with Atopic dermatitis is based on body weight:
|
Body Weight of Patient |
Initial dose |
Subsequent Doses (every other week) |
|
Less than 60kg |
400mg (two 200mg injections) |
200mg |
|
60kg or more |
600mg (two 300mg injections) |
300mg |
Recommended dose in adult and adolescent patients with Asthma For Asthma, the recommended dose of Dupixent for adult and adolescents patients (12 years of age and older) is:
- For patients with Severe asthma and who are on Oral corticosteroids or for patients with Severe asthma and comorbid moderate-to-severe Atopic dermatitis, an initial dose of 600 mg (two 300 mg injections), followed by 300 mg every other week administered as subcutaneous injection.
- For all other patients, an initial dose of 400 mg (two 200 mg injections), followed by 200 mg every other week administered as subcutaneous injection. Dupixent is given by injection under your skin (subcutaneous injection). You and your Doctor or nurse should decide if you should inject Dupixent yourself.
Contraindications
Do not use Dupixent
- If you are allergic to Dupilumab or any of the other ingredients of this medicine (listed in section 6).
- If you think you may be allergic, or you are not sure, ask your Doctor, Pharmacist or nurse for advice before using Dupixent
Warning and Precautions
Talk to your Doctor, Pharmacist or nurse before using Dupixent:
Dupixent is not a rescue medicine and should not be used to treat a sudden asthma attack. Allergic reactions Very rarely, Dupixent can cause serious side effects, including allergic (hypersensitivity) reactions and anaphylactic reaction. You must look out for signs of these conditions (i.e. breathing problems, swelling of the face, mouth, and tongue, fainting, dizziness, feeling lightheaded (low blood pressure), fever, general ill feeling, swollen lymph nodes, hives, itching, joint pain, skin rash) while you are taking Dupixent. Stop using Dupixent and tell your doctor or get medical help immediately if you notice any signs of an allergic reaction. Such signs are listed under “Serious side effects” in section 4.
Eosinophilic conditions Rarely patients taking an Asthma medicine may develop inflammation of blood vessels or lungs due to an increase of certain white blood cells (Eosinophilia). It is not known whether this is caused by Dupixent. This usually, but not always, happens in people who also take a Steroid medicine which is being stopped or for which the dose is being lowered. Tell your doctor immediately if you develop a combination of symptoms such as a flu-like illness, pins and needles or numbness of arms or legs, worsening of pulmonary symptoms, and/or rash. Parasitic (intestinal parasites) infection Dupixent may weaken your resistance to infections caused by parasites. If you already have a Parasitic infection it should be treated before you start treatment with Dupixent. Check with your Doctor if you have Diarrhea, gas, upset stomach, greasy stools, and Dehydration which could be a sign of a Parasitic infection. If you live in a region where these infections are common or if you are travelling to such a region check with your doctor. Asthma If you have asthma and are taking Asthma medicines, do not change or stop your Asthma medicine without talking to your Doctor.
Talk to your doctor before you stop Dupixent or if your Asthma remains uncontrolled or worsens during treatment with this medicine. Eye problems (if you have Atopic dermatitis) Talk to your Doctor if you have any new or worsening eye problems, including eye pain or changes in vision.
Adverse reaction
Dupixent can cause serious side effects, including very rare allergic (hypersensitivity) reactions, including anaphylactic reaction; the signs of allergic reaction or anaphylactic reaction may include:
breathing problems, swelling of the face, mouth, and tongue, fainting, dizziness, feeling lightheaded (low blood pressure), fever, general ill feeling, swollen lymph nodes, hives, itching, joint pain skin rash,
If you develop an allergic reaction, stop using Dupixent and talk to your Doctor right away.
Other side effects
Very Common (may affect more than 1 in 10 people) Atopic dermatitis and Asthma:injection site reactions (i.e. redness, swelling, and itching)
Common (may affect up to 1 in 10 people) Atopic dermatitis only:Headache, eye dryness, redness and itching, eyelid itching, redness and swelling eye infection, cold sores (on lips and skin)
Drug interactions
Tell your Doctor or Pharmacist
- If you are using, have recently used or might use any other medicines.
- If you have recently had or are due to have a vaccination.
Do not stop or reduce your Asthma medicines, unless instructed by your Doctor. These medicines (especially ones called corticosteroids) must be stopped gradually, under the direct supervision of your Doctor and dependent on your response to Dupixent.
Pregnancy and Breast-feeding
- If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine. The effects of this medicine in pregnant women are not known; therefore it is preferable to avoid the use of Dupixent in pregnancy unless your Doctor advises to use it.
- If you are breast-feeding or are planning to breast-feed, talk to your Doctor before using this medicine. You and your Doctor should decide if you will breastfeed or use Dupixent. You should not do both.
Driving and Using machines
Dupixent is unlikely to influence your ability to drive and use machines.
- Always read the full prescribing information.
- Healthcare professionals are asked to report any suspected adverse reactions to the Egyptian Pharmacovigilance Center (EPVC).
- Egyptian drug authority dupixent leaflet approval date 6/1/2021.