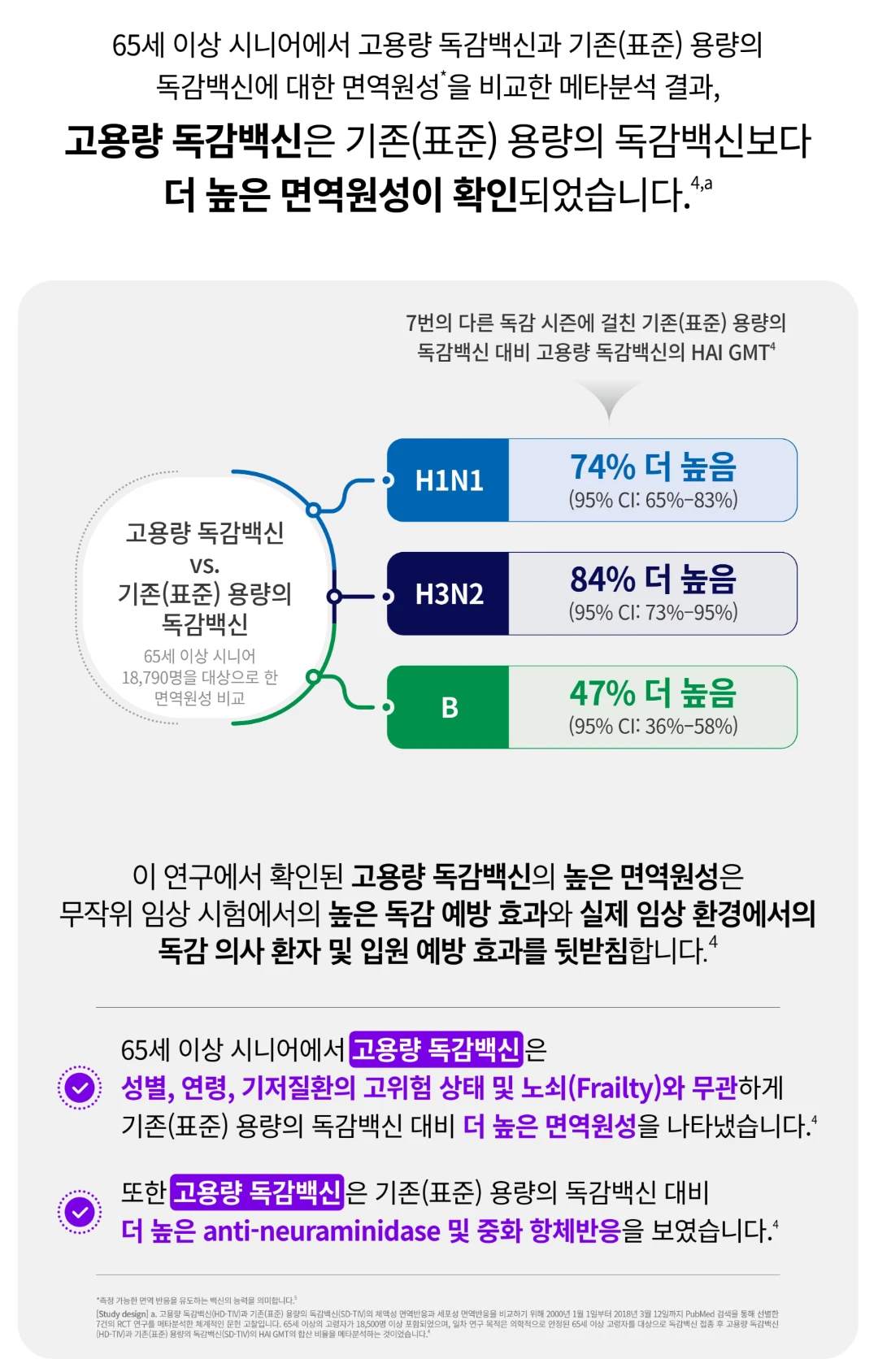
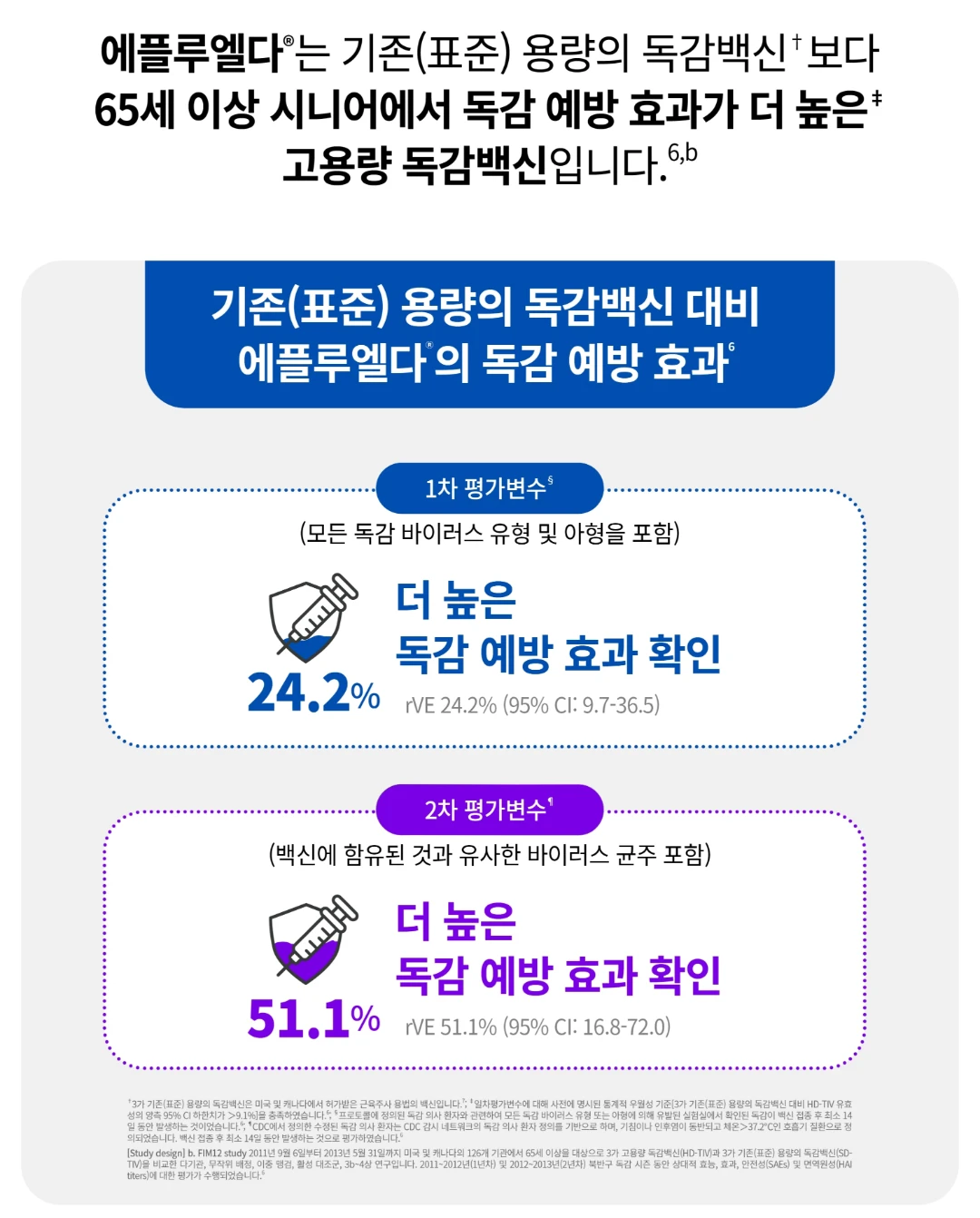
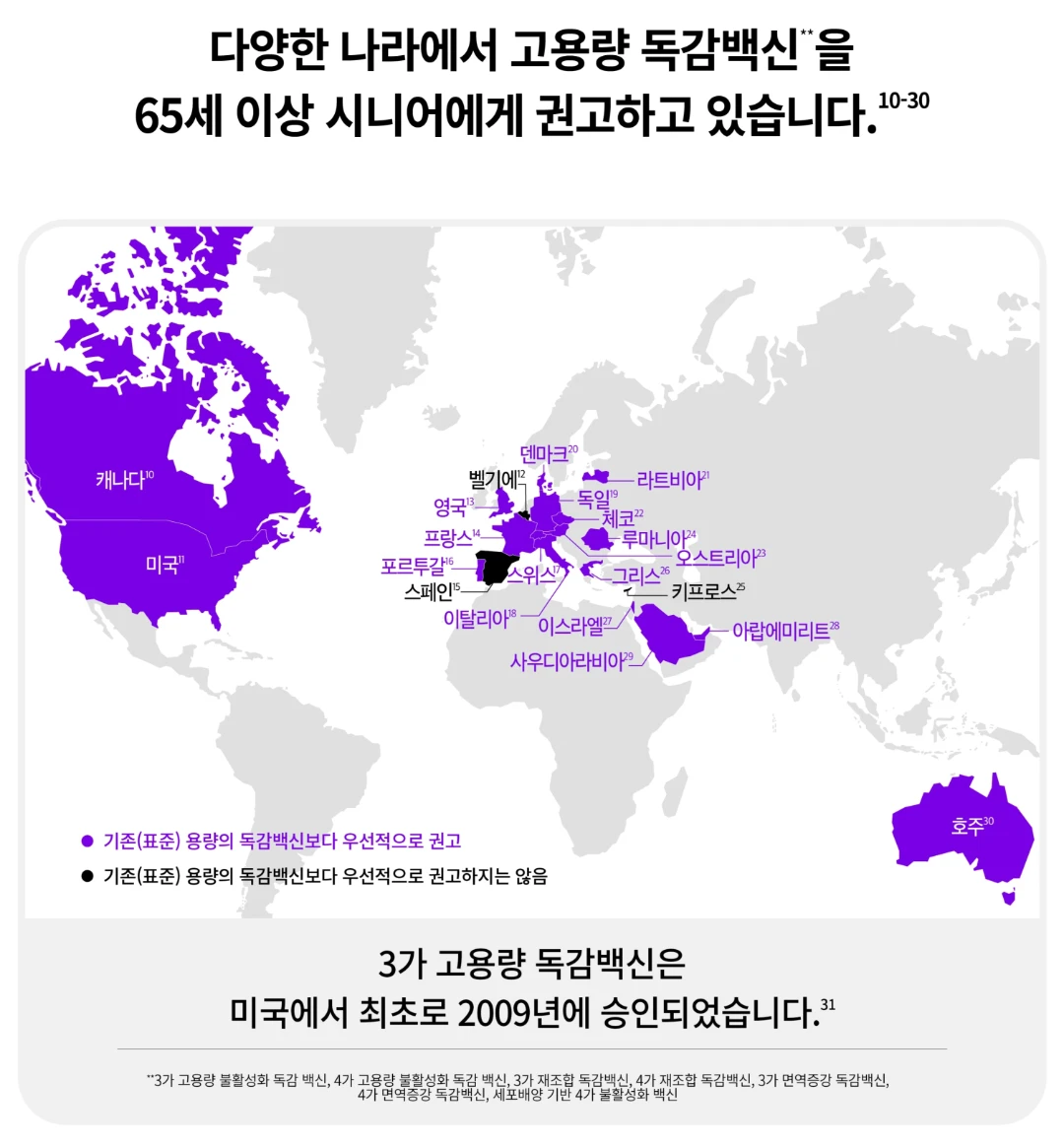
독감, 백신, 에플루엘다, 에플루엘다가격, 에플루엘다효능, 독감에플루엘다, 고용량독감백신, 선택형백신, 독감백신, 예방접종, 감기에좋은음식, 기침멈추는법, 기침에좋은차, 독감이빨리낫는법, 기관지에좋은음식, 기침가래에좋은약, 감기빨리낫는법, 기침계속, 기침가래, 예방접종증명서, 기침, 기관지, 백신추천, 예방접종확인
CDC, Centers for Disease Control and Prevention; CI, confidence interval; GMT, geometric mean titer; HA, hemagglutinin; HAI, hemagglutination inhibition; HD-TIV, high-dose trivalent influenza vaccine; RCT, randomized controlled trial; rVE, relative vaccine effectiveness; SAEs, serious adverse events; SD-TIV, standard-dose trivalent influenza vaccine; VAERS, vaccine adverse event reporting system.
- 의약품안전나라 의약품통합정보시스템. 에플루엘다프리필드시린지. Available at https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetailCache?cacheSeq=202500885aupdateTs2025-04-23%2008:10:30.831909b. Accessed on Jul. 29, 2025.
- Diaco M, et al. Vaccine. 2021;39 Suppl 1:A1-A5.
- 의약품통합정보시스템. 의약품제품정보. 박씨그리프주. Available at https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetailCache?cacheSeq=202500839aupdateTs2025-06-30%2014:38:09.0b. Accessed on Jul. 30, 2025.
- Samson SI, et al. Expert Rev Vaccines. 2019;18:295-308.
- 식품의약품안전처. 식품의약품안전 평가원. 백신 임상평가 가이드라인 (2017.6).
- DiazGranados CA, et al. N Engl J Med. 2014;371(7):635-645.
- DiazGranados CA, et al. N Engl J Med. 2014;371(7):635-645(Protocol).
- DiazGranados CA, et al. Vaccine. 2013;31(6):861-866.
- Moro PL et al. Vaccine. 2020;38(37):5923-5926.
- Government of Canada. Influenza vaccines: Canadian Immunization Guide. Available at https://www.Canada.ca/en/public-health/services/publications/healthy-living/Canadian-immunization-guide-part-4- active-vaccines/page-10-influenza-vaccine.html#a5.2. Accessed on Apr. 07, 2025.
- Grohskopf LA, et al. MMWR Recomm Rep. 2024;73(5):1-25.
- Belgium. Conseil Superieur de la Santé- 2024-2025. Available at https://www.vivalis.brussels/sites/default/files/2024-09/Vaccination%20contre%20la%20grippe%20saisonni%C3%A8re%20-%20saison%20 hivernal%202024-2025.pdf. Accessed on Apr. 07, 2025.
- JCVI statement on influenza vaccines for 2025 to 2026 – GOV. Available at https://www.gov.uk/government/publications/flu-vaccines-2025-to-2026-jcvi-advice Accessed on Jul. 08, 2025.
- France Haute Autorité de Santé – Vaccination Available at https://www.has-sante.fr/jcms/p_3604446/fr/vaccination-contre-la-grippe-saisonniere-des-personnes-de-65-ans-et-plus-place-des-vaccins- eflueldaet-fluad Accessed on Jul. 08, 2025.
- ECDC. Survey report on national seasonal influenza vaccination recommendations and coverage rates in EU/EEA countries. Data from the 2024 ECDC influenza survey, 2021–22 to 2023–24 influenza seasons.
- Portugal: DGS. 2024. Available at https://www.portugal.gov.pt/download-ficheiros/ficheiro.aspx?v=%3d%3dBQAAAB%2bLCAAAAAAABAAzNDEyMQMATV5VdgUAAAA%3d. Accessed on Apr. 07, 2025.
- Switzerland. Available at https://backend.bag.admin.ch/fileservice/sdweb-docs-prod-bagadminch-files/files/2025/05/08/5f1f0792-3b3d-4aab-9ed8-35ffe6cfd44b.pdf. Accessed on Aug. 04, 2025.
- Italy Ministero della Salute. OGGETTO: Prevenzione e controllo dell’influenza: raccomandazioni per la stagione 2024-2025. Available at https://www.trovanorme.salute.gov.it/norme/ renderNormsanPdf?anno=2024&codLeg=100738&parte=1%20&serie=null. Accessed on Aug. 04, 2025.
- ROBERT KOCH INSTITUT. Epidemiologisches Bulletin 2025.4.
- Denmark. Health Authority. 2023. Available at https://www.sst.dk/-/media/Udgivelser/2023/Vaccination/2023-2024/Foreloebig-indstilling-til-maalgrupper-og-vaccinetyper-til-indkoeb.ashx. Accessed on Apr. 07, 2025.
- Latvia Veselības ministrija. Rekomendētā vakcinācija un piemērotākās vakcīnas PIEAUGUŠAJIEM. Available at https://www.vm.gov.lv/lv/imunizacijas-valsts-padome?utm_source=https%3A%2F%2Fwww.go ogle.com%2F. Accessed on Jun. 23, 2025.
- Czech Republic. NICO. 2022. Available at https://www.vakcinace.eu/doporuceni-a-stanoviska/doporuceni-ceske-vakcinologicke-spolecnosti-cls-jep-k-ockovani-proti-chripce-3. Accessed on Apr. 07, 2025.
- Austria. Bundesministerium Arbeit, Soziales, Gesundheit, Pflege und Konsumentenschuts. Impfplan Österreich 2024/2025. Version 1.1 vom 18.12.2024. Available at https://www.sozialministerium.at/ Themen/Gesundheit/Impfen/Impfplan-%C3%96sterreich.html. Accessed on Apr. 07, 2025.
- Romania: MoH. 2023. Available at https://legislatie.just.ro/Public/DetaliiDocument/274834. Accessed on Apr. 07, 2025.
- Cyprus. MoH 2023 National Advisory Committee. Available at https://cyprus-mail.com/2023/09/22/simultaneous-covid-and-flu-vaccinations-to-begin-from-october/. Accessed on Apr. 07, 2025.
- Greece. National Adult Immunization Program 2025 Schedule and Recommendations Available at https://www.moh.gov.gr/articles/health/dieythynsh-dhmosias-ygieinhs/emboliasmoi/ethniko- programmaemboliasmwn-epe-enhlikwn/13219-ethniko-programma-emboliasmwn-enhlikwn-2025-xronodiagramma-kai-systaseis?dl=1. Accessed on Jul. 08, 2025.
- Israel. MoH. 2023. Available at https://www.gov.il/BlobFolder/reports/committee-infectious-diseases-vaccines-summary-2023/he/files_committees_committee-infectious-diseases-vaccines_ CMV_08022023.pdf. Accessed on Apr. 07, 2025.
- United Arab Emirates: ADPHC. 2023. Available at https://www.doh.gov.ae/-/media/A350695766F547B4AAEF90C30270EDE4.ashx. Accessed on Apr. 07, 2025.
- ECONOMIST IMPACT. Unmasking the risk and burden of seasonal influenza in the Middle East. Available at https://www.researchgate.net/publication/392160314_Unmasking_the_risk_and_bu rden_of_seasonal_influenza_in_the_Middle_East_Strengthening_prevention_and_control_strategies_for_a_healthier_tomorrow_Unmasking_the_risk_and_burden_of_seasonal_influenza_in_t/ link/683707bc6b5a287c304716f0/download?_tp=eyJjb250ZXh0Ijp7ImZpcnN0UGFnZSI6InB1YmxpY2F0aW9uIiwicGFnZSI6InB1YmxpY2F0aW9uIn19. Accessed on Aug. 04, 2025.
- A joint Australian, State and Territory Government Initiative. Available at https://www.health.gov.au/sites/default/files/2025-03/2025-influenza-vaccination-program-advice-for-health-professionals_0.pdf. Accessed on Apr. 07, 2025.
- Lee JKH, et al. Vaccine X. 2023;14:100327.
에플루엘다 프리필드시린지(인플루엔자분할백신)
제품정보는 LINK를 통해 확인하시기 바랍니다. (문안개정년월일 2025.04.14)
광고심의필 2025-1774-105802
※ 백신은 의사가 처방해야 하는 의약품으로 의사와 상의하십시오.
※ 부작용이 있을 수 있으니 ‘사용상의 주의사항’을 잘 읽고, 의사·약사와 상의하십시오. 인터넷 의약품 판매행위는 불법입니다.
주식회사 사노피-아벤티스 코리아, 서울특별시 서초구 반포대로 235 (우편번호 06578) | 이용약관 | 법적고지 | 쿠키정책 |
대표자 : 배경은
사업자등록번호 : 220-81-00544
대표전화번호: (02)2136-9000
팩스번호: 02-2136-9588
문의사항은 잠재적인 이상사례 보고를 위해 모니터링 되고, 이상사례의 추가정보를 위해 회사로부터 연락 받으실 수 있습니다.
문의사항은 법 및 규정 준수의 의무를 위해 회사가 수집합니다.
MAT-KR-2501438-2.0-09/2025