An easy guide to use Nirsevimab
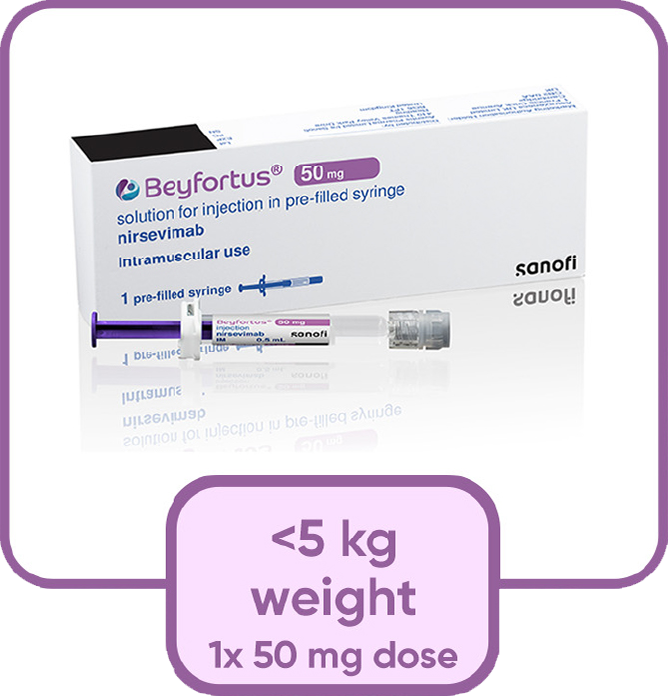
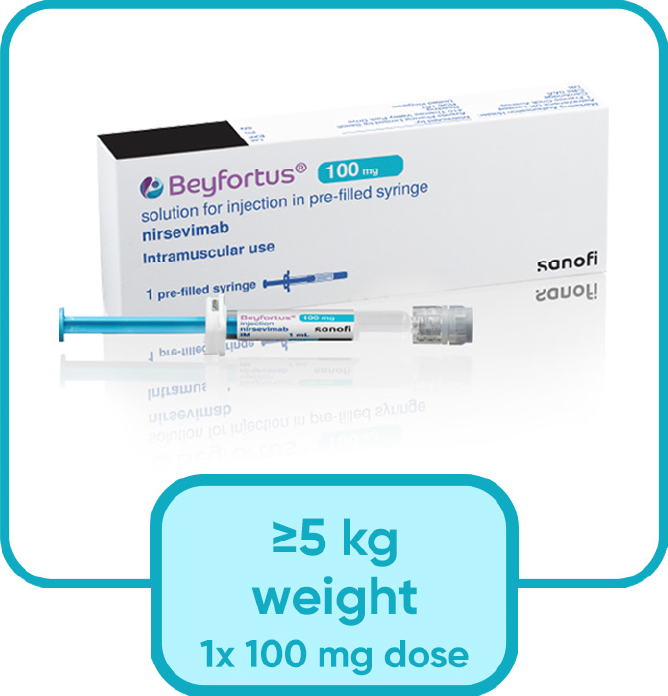
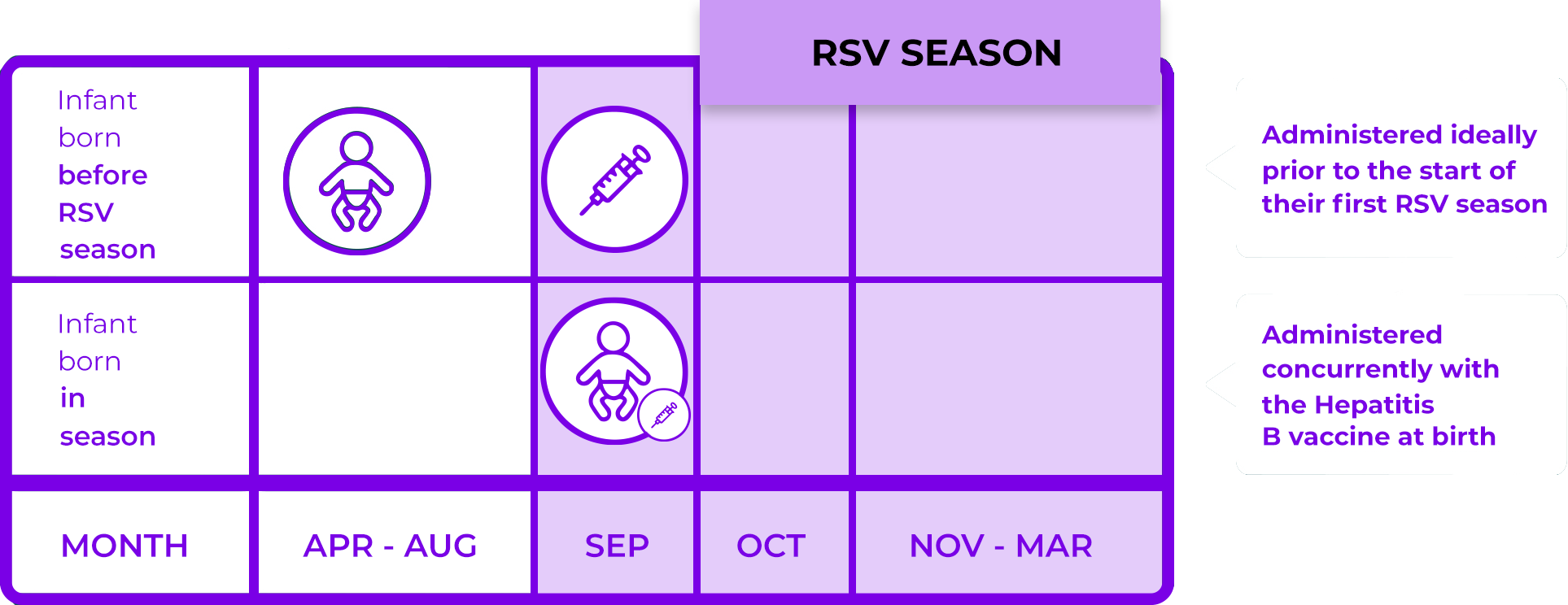
Nirsevimab is given for infants entering their first RSV season as a single injection based on infant's weight at the time of dosing
How to use Nirsevimab
Nirsevimab comes in a ready-to-use pre-filled syringes with no need to reconstitute
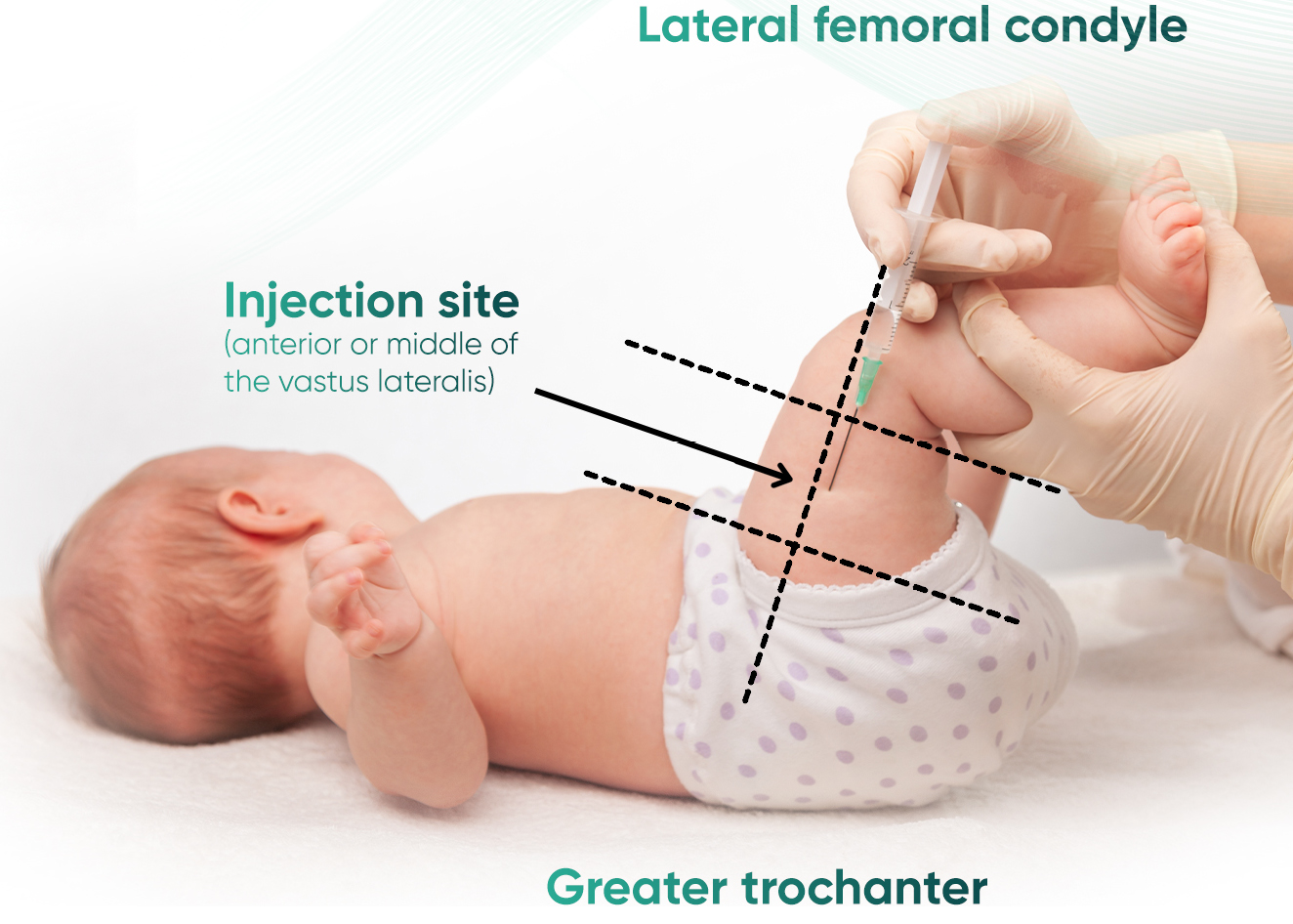
Given as an intramuscular injection, preferably into the anterolateral aspect of the thigh
Can be administered concomitantly with childhood vaccines
How to store Nirsevimab

- Store in a refrigerator (2-8°C)
- Do not freeze
- Do not shake or expose to direct heat
- Keep the pre-filled syringe in the outer carton in order to protect from light

- Nirsevimab has a shelf life of 3 years
- Nirsevimab may be kept at room temperature (20°C-25°C) when protected from light for a maximum of 8 hours. After this time, the syringe must be discarded
When to administer Nirsevimab
The duration of protection afforded by Nirsevimab is at least 5 months.
Illustrative example only. For full dosing information, consult the Summary of Product Characteristics.
Luer Lock Syringe Components
-5_03.png/jcr:content/Gauide-dosage-english-(1)-5_03.png) Step 1: Holding the Luer lock in one hand, unscrew the syringe cap by twisting it counter-clockwise with the other hand.
Step 1: Holding the Luer lock in one hand, unscrew the syringe cap by twisting it counter-clockwise with the other hand.
-5_06.png/jcr:content/Gauide-dosage-english-(1)-5_06.png) Step 2: Attach a Luer lock needle to the pre-flled syringe by gently twisting the needle clockwise onto the pre-filled syringe until slight resistance is felt. (16mm-25mm)
Step 2: Attach a Luer lock needle to the pre-flled syringe by gently twisting the needle clockwise onto the pre-filled syringe until slight resistance is felt. (16mm-25mm)
-5_08.png/jcr:content/Gauide-dosage-english-(1)-5_08.png) Step 3: Hold the syringe body with one hand and carefully pull the needle cover straight off with the other hand.
Step 3: Hold the syringe body with one hand and carefully pull the needle cover straight off with the other hand.
-5_10.png/jcr:content/Gauide-dosage-english-(1)-5_10.png) Step 4: Administer the entire contents of the Nirsevimab pre-filled syringe as an IM injection, preferably in the anterolateral aspect of the thigh. The gluteal muscle should not be used as an injection site because of the risk of damage to the sciatic nerve.
Step 4: Administer the entire contents of the Nirsevimab pre-filled syringe as an IM injection, preferably in the anterolateral aspect of the thigh. The gluteal muscle should not be used as an injection site because of the risk of damage to the sciatic nerve.
-5_12-copy.png/jcr:content/Gauide-dosage-english-(1)-5_12%20copy.png) Step 5: Discard syringe into a sharps container.
Step 5: Discard syringe into a sharps container.
SANOFI, Kingdom of Saudi Arabia, PO Box 9874, Jeddah 21423, K.S.A. Tel.: +966-12-669-3318
For further medical information, please contact:
KSA / +966-12-669-3318 or email: ksa.medicalinformation@sanofi.com
To report adverse events please contact: The National Pharmacovigilance Center (NPC): Call Center: 19999, E-mail: npc.drug@sfda.gov.sa, Website: https://ade.sfda.gov.sa/ And Sanofi Pharmacovigilance Department: Phone: +966-544-284-797, E-mail: Ksa_pharmacovigilance@sanofi.com
To report any product technical complaints, kindly contact: email: quality.greatergulf@sanofi.com
Sanofi Gulf, One JLT, 3rd floor, JLT. PO BOX - 53899, Dubai - UAE For further medical information, please contact: For UAE ✆ 800 MEDICAL Toll-Free Number. For all Gulf countries ✆ +97145503863 or email: medical-information.gulf@sanofi.com. To report an adverse event or drug reaction, please contact us on 24/7 Pharmacovigilance:+971561747001 | Email: gulf.pharmacovigilance@sanofi.com. To report any product technical complaints, kindly contact: quality.greatergulf@sanofi.com