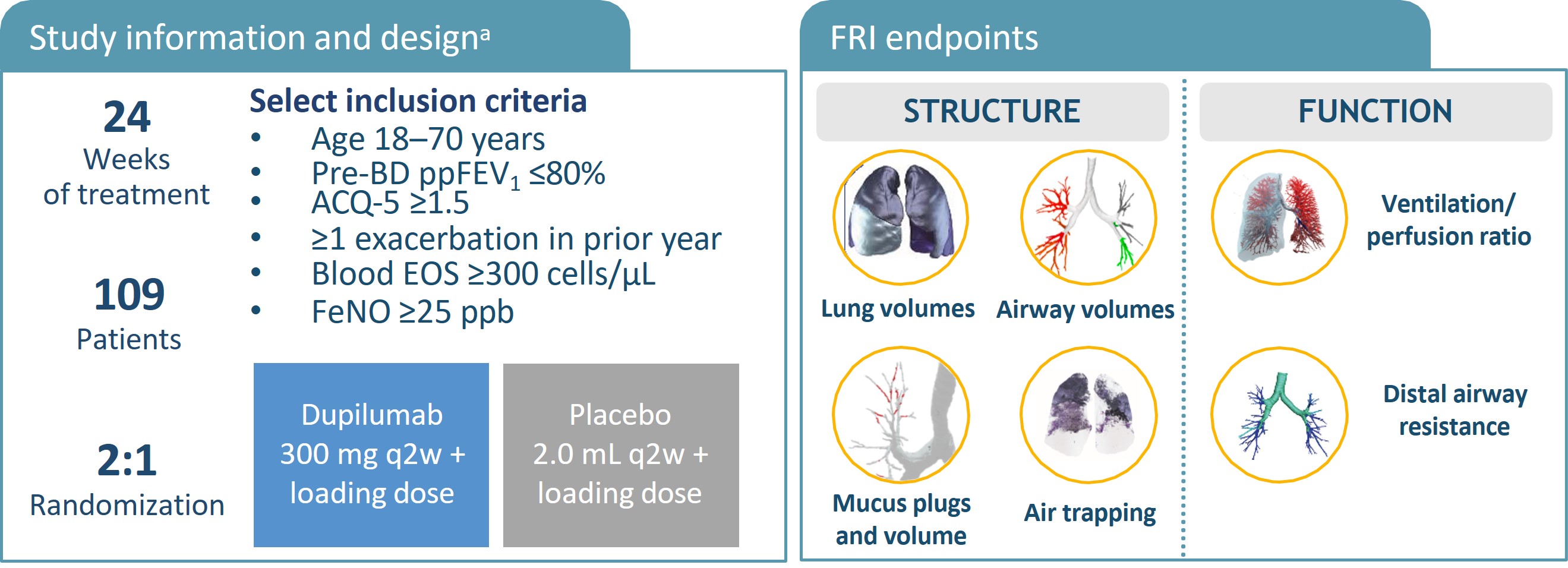
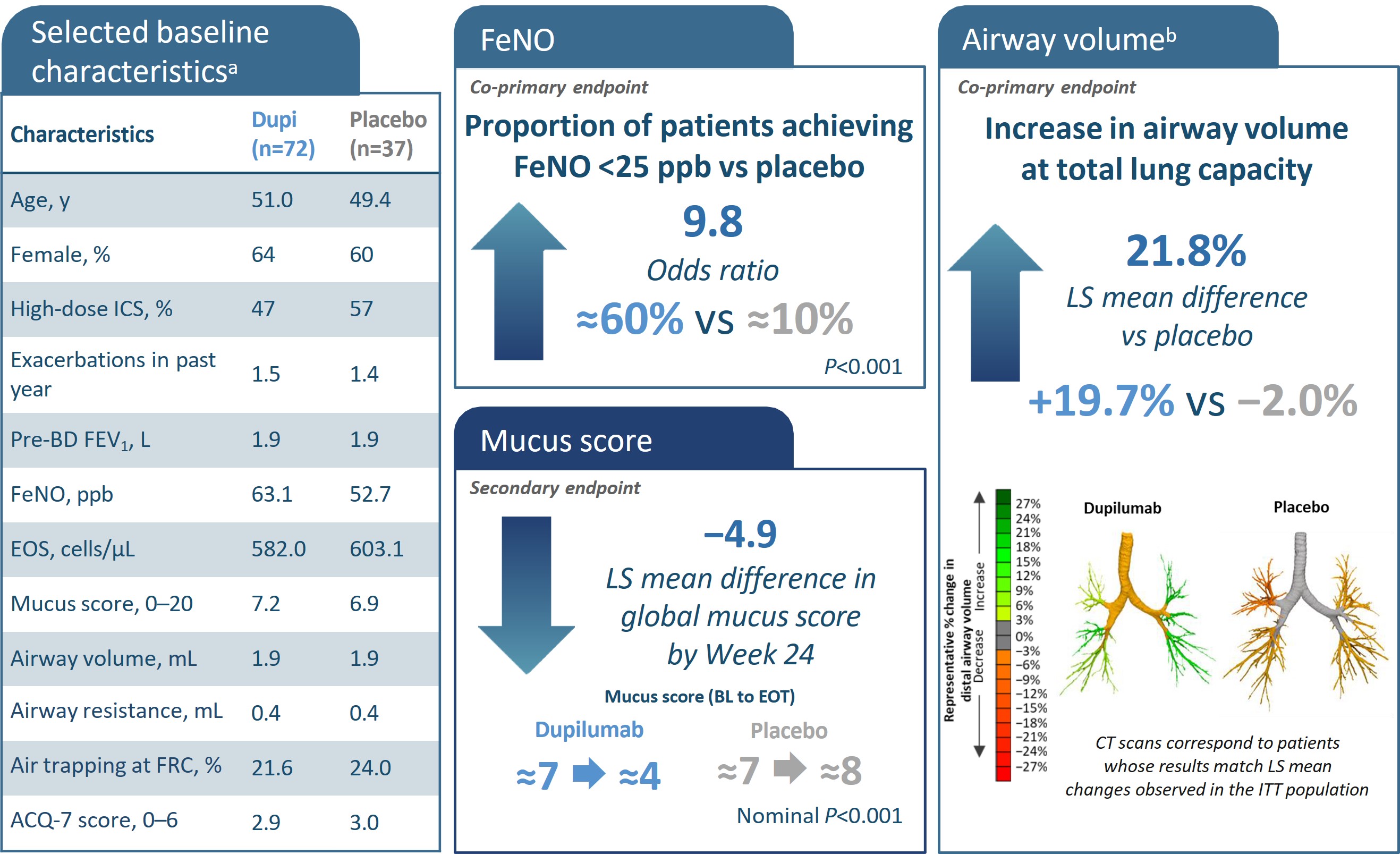
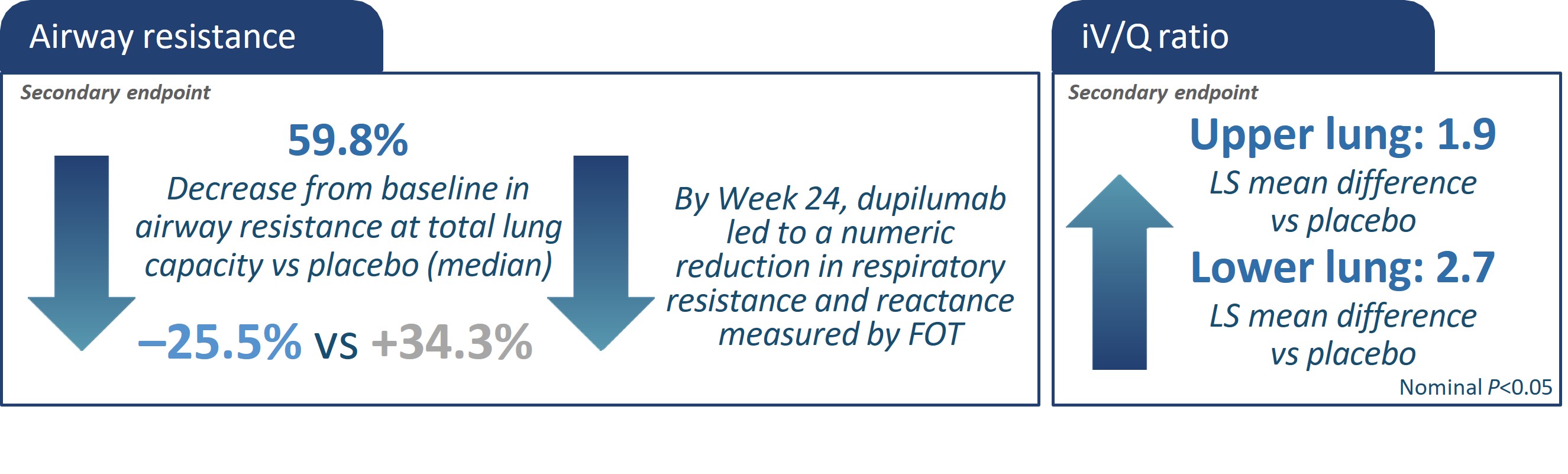
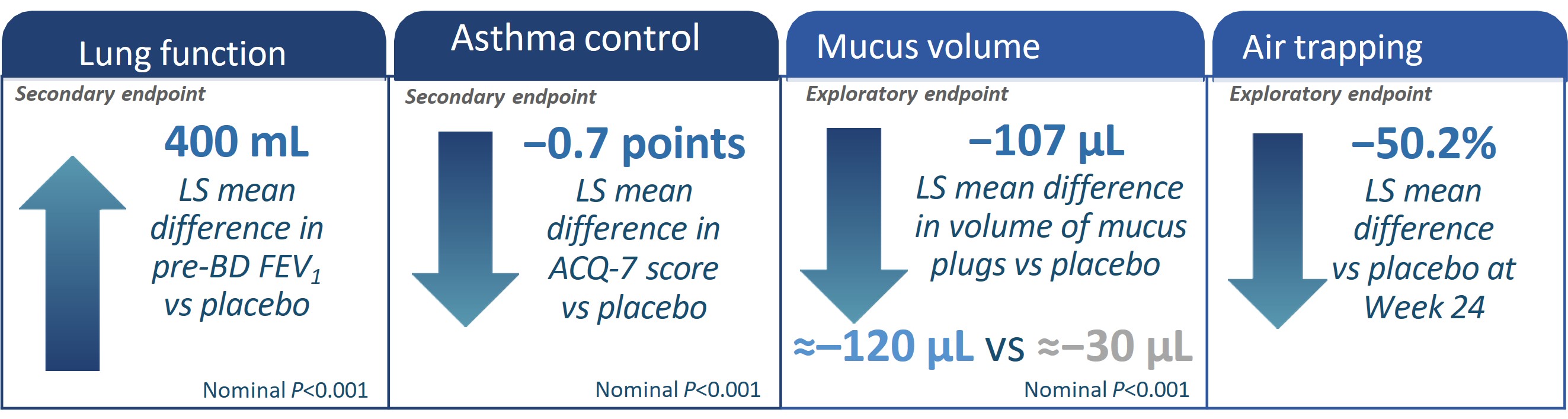
{
event: "article_read",
name: `VESTIGE study`,
author: ``,
tags: `Asthma`,
publication_date: ``,
interaction_type: "content"
}
Effect of Dupilumab on Exhaled Nitric Oxide, Mucus

VESTIGE study
Effect of Dupilumab on Exhaled Nitric Oxide, Mucus
Plugs, and Functional Respiratory Imaging in Patients
With Type 2 Asthma (VESTIGE): A Randomised,
Double-blind, Placebo-controlled, Phase 4 Trial
Castro M, et al. Lancet Respir Med.2025 Online first

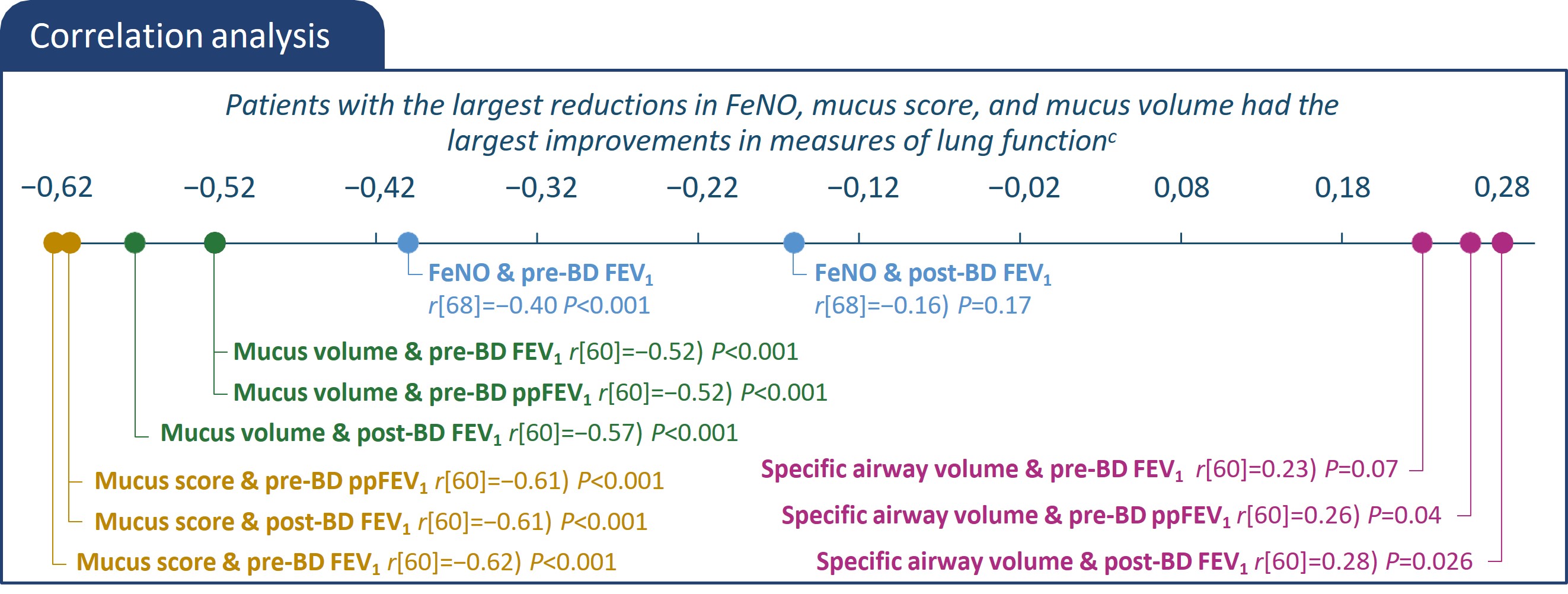
*All characteristics are mean unless otherwise noted. bAs the primary imaging endpoint did not reach statistical significance (P>0.05), all subsequent endpoints were reported
using nominal p-values. cThe statistical significance represents the correlations between 2 parameters.
ACQ, asthma control questionnaire; BD, bronchodilator; CT, computed tomography; EOS, eosinophils; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1
second; FOT, forced oscillation technique FRI, functional residual imaging; ICS, inhaled corticosteroids; ITT, intention-to-treat; iV/Q, image-based ventilation-perfusion ratio; LS,
least squares; ppFEV1, percent predicted forced expiratory volume in 1 second; q2w, every 2 weeks.
MAT-BH-2500106/V1/FEB2025