Multiple copies of chromosome 1 (1q+) is a frequent CA in MM
- MM is characterized by CAs in plasma cells1
- Frequent gains include 1q, 6p and 11q1
- 1q+ is one of the most common CAs in MM

1q+ abnormalities are defined by copy number
Chromosome 1q abnormalities terminology3
| +1q | Additional copies of any part of the long arm of chromosome 1 (1q), irrespective of copy number or DNA segment gained |
| Gain(1q) | Gain of only 1 extra copy of chromosome 1q (3 total copies) |
| Amp(1q) | “Amplification” of 1q, with 2 additional copies of chromosome 1q (4 or more total copies) |
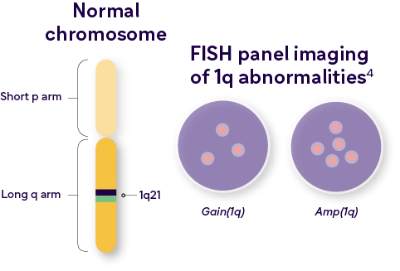
In MM, 1q+ is frequently denoted as 1q21+, which refers to a specific location – region 2, band 1 on the long arm of chromosome 1
Overexpression of certain genes in the 1q arm may drive pathogenesis and drug resistance4,5
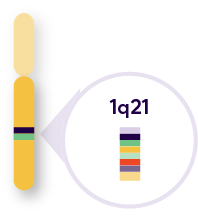
Key genes located in the 1q21 band:
|
Resistance to PIs through the upregulation of the proteasome genes, including PSMD6 |
|
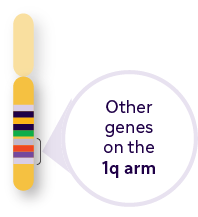
Other key genes outside of the 1q21 band include:
|
Resistance to drugs that rely mainly on CDC through upregulation of complement inhibitors, such as CD557 |
|
1q+ terminology when co-occurring with/without other high-risk cytogenetic abnormalities (HRCA)8

1q+ increases throughout the MM disease course4,9

Prognosis worsens with increasing copy number

Incorporating 1q+ testing into clinical practice remains challenging
New staging systems, such as R2-ISS, MASS and R-ISS-1q,12–14 are including gain/amp(1q), alongside other high-risk factors when defining disease stage, in order to refine predictions of clinical outcomes and highlight the prognostic impact of gain/amp(1q)

Although most guidelines include 1q+ in their HRCA definitions, the recommendations for FISH panel testing for 1q+ are inconsistent and there is little treatment guidance specific to gain/amp(1q)15–17

IMWG guidelines include gain/amp(1q) in their definition of HRCAs, but do not include it as part of their recommended routine FISH panel15
CA, cytogenetic abnormality; CDC, complement-dependent cytotoxicity; FISH, fluorescence in situ hybridization; HRCA, high-risk cytogenetic abnormality; IMWG, International Myeloma Working Group; ISS, international staging system; LDH, lactate dehydrogenase; MASS, The Mayo Additive Staging System; MM, multiple myeloma; MRD, minimal residual disease; NDMM, newly-diagnosed multiple myeloma; OS, overall survival; PFS, progression-free survival; PI, protease inhibitor; R-ISS, Revised International Staging System; RRMM, relapsed/refractory multiple myeloma.
- Hassan H and Szalat R. Appl Clin Genet 2021;14:241–54;
- Hanumura I, et al. Int J Hematol 2022; 115:762–777;
- Schmidt TM, et al. Blood Cancer J 2021;11:83;
- Hanamura I. Cancers 2021;13:256;
- Bisht K, et al. Exp Rev Haem 2021;14:1099–114;
- Garcia J, et al. Cells 2021;10:1360;
- Golay J and Taylor R. Antibodies 2020;9:58;
- Harrison SJ, et al. Br J Haematol 2021;194:120–31;
- Hanamura I, et al. Blood 2006;108:1724–32;
- D’Agostino M, et al. Presented at IMW 2021. Abstract 1062754;
- Costa LJ et al. Presented at ASH 2021. Abstract 481 (oral presentation);
- Hu X, et al. Ann Hematol. 2022;101:369–378;
- Wang Y, et al. Cancer 2023. doi: 10.1002/cncr.34641;
- Hongying Y et al. Presented at ASH 2022. Abstract 4518;
- Weinhold N, et al. Haematologica 2021;106:2754–8;
- Abdallah N, et al. Blood Cancer J 2022;12:21;
- D’Agostino M, et al. Presented at ASH 2020. Abstract 1329;
- Sonneveld P et al. Blood 2016;127:2955–62;
- Dimopoulos MA, et al. Ann Oncol 2021;32:309–22;
- mSMART treatment of Newly Diagnosed Myeloma https://www.msmart.org/mm-treatment-guidelines.
MAT-AE-2300647-V1-Nov-23