Proven Safety of Dupixent® against Type 2 Inflammation

Dupixent has emerged as a significant therapeutic option for the treatment of various type 2 inflammatory diseases. As a fully human monoclonal antibody that inhibits interleukin-4 (IL-4) and interleukin-13 (IL-13) signaling, Dupixent offers a targeted approach to managing conditions such as Atopic Dermatitis, Asthma, Chronic Rhinosinusitis with Nasal Polyposis, and Eosinophilic Esophagitis and COPD1.
Mechanism of Action and Safety Implications
Dupixent's unique action as an immunomodulator, rather than a broad immunosuppressant, supports its positive safety profile. By targeting IL-4 and IL-13 pathways specifically, Dupixent provides immune response control without significantly compromising immune defenses, which may lower the risk of serious infections often seen with other immunosuppressive treatments.
Inhibition of IL-4 and IL-13 signaling has a substantial impact on Type 2 inflammation diseases as it helps reduce the inflammation by2:
- Helping reduce Itch: May break itch-scratch cycle and reduce nerve sensitization
- Helping achieve nodule clearance: Reduce skin fibrosis
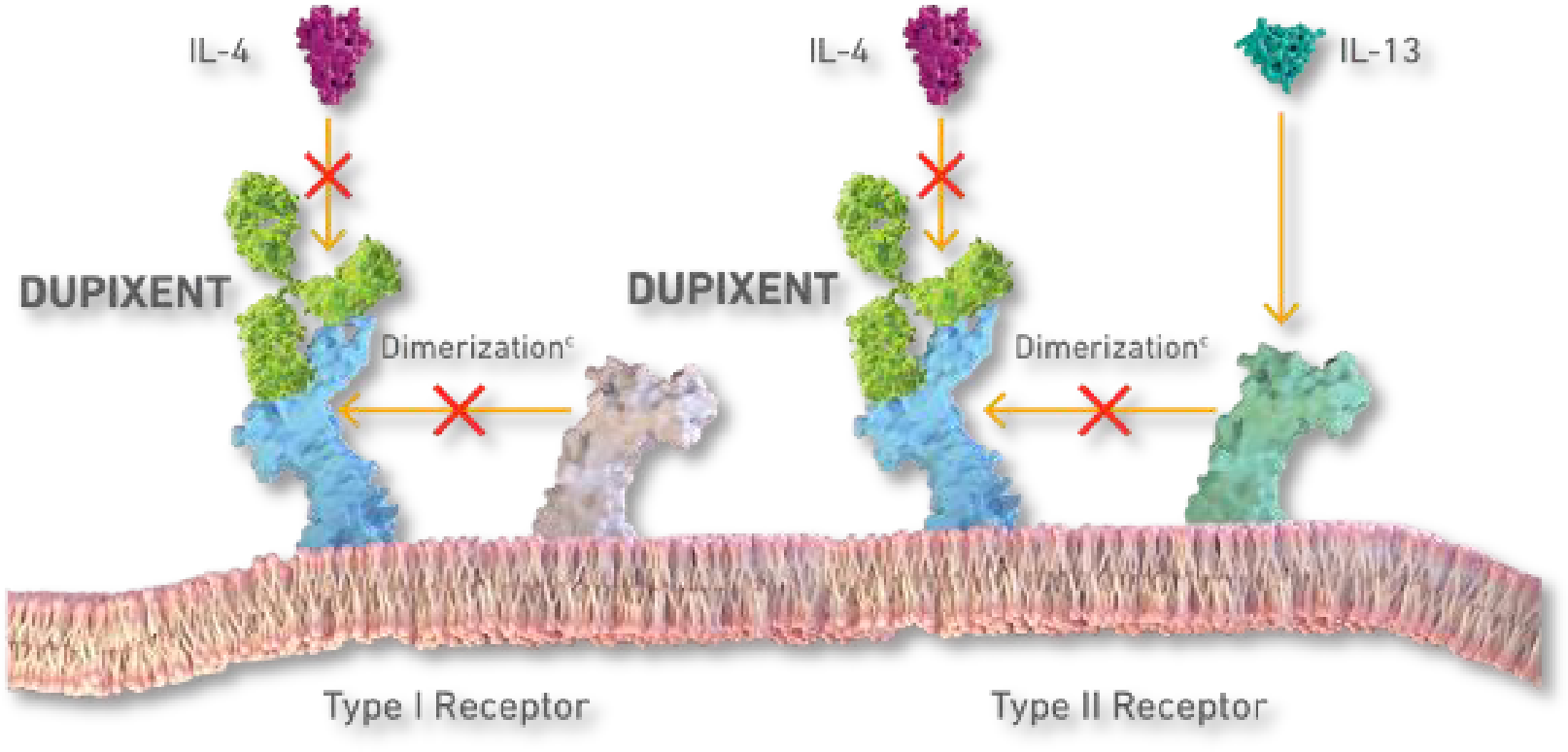
Figure 1: DUPIXENT is the only dual inhibitor of IL-4 and IL-13 signaling2
Long-Term Safety Data
Dupixent's safety has been confirmed through studies extending up to five years, covering over 800,000 patients globally.
This trial, involving 2,677 patients, found that the safety profile remained consistent with earlier controlled trials1.
Common Adverse Events
Common adverse effects seen across trials include injection site reactions, conjunctivitis (more common in those with atopic dermatitis), nasopharyngitis, headache, and back pain.
In recent COPD trials, adverse effects with a frequency of ≥5% included COVID-19 infection and diarrhea compared to placebo3,4.
Safety by Indication
| AD
| 2% | of TEA leading to permanent Dupixent discontinuation vs 8% for placebo5 |
| Asthma
| 3% | of TEA leading to permanent Dupixent discontinuation vs 6% for placebo6 |
| CRSwNP
| 4% | of TEA leading to permanent Dupixent discontinuation vs 11% for placebo7 |
| EOE
| Most TAEAs mild or moderate8 | |
| PN
| Similar incidences of TEAEs in patients treated with DUPIXENT vs placebo arm (64% and 57% respectively)9 | |
Regulatory Perspective and Approvals
Regulators like the U.S. FDA and the European Medicines Agency (EMA) have reviewed comprehensive safety data, concluding that Dupixent's benefits outweigh associated risks, which has led to approval in over 60 countries. The consistent safety profile across various inflammatory conditions has supported regulatory endorsements1.
Special Considerations

Pediatric Use:
Dupixent has shown a favorable safety profile in younger patients, receiving approval for certain conditions in patients as young as 6 months old1.
Minimal Immunosuppression:
Unlike many biologic therapies, Dupixent does not broadly suppress the immune system, contributing to its positive long-term safety profile1.

Monitoring Needs:
Dupixent does not require initial or ongoing laboratory testing, highlighting its low risk of serious systemic effects.
Clinical trial and post-market data affirm Dupixent's consistent and favorable safety profile across multiple type 2 inflammatory diseases.
Its targeted action and generally mild side effects underscore its strong benefit-risk profile.
Dupixent's safety has been maintained across different age groups and in extended use, with no significant safety issues arising in long-term studies.
Current evidence supports its use as a safe and effective option for patients with type 2 inflammatory diseases.
- https://www.sanofi.com/en/media-room/press-releases/2024/2024-02-23-06-00-00-2834219
- https://www.dupixenthcp.com/prurigo-nodularis/about/mechanism-of-action
- https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-11-05-05-00-2944239
- https://www.sanofi.com/en/media-room/press-releases/2024/2024-05-31-05-00-00-2891259
- Regeneron Pharmaceuticals. CHRONOS CTR report full body. [p190/table50, p201/table56, p205/table57]
- Castro, M., et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma New England Journal of Medicine. (2018). [p8/table2, p9/col1-col2/h1-h3]
- Bachart C, Han JK, Desrosiers M, et al. (2019) "Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials". Lancet. 2019 Sep 19. (Table S12, Table S13)
- Dellon E., et al 2022. Dupilumab in Adult and Adolescent Patients with Eosinophilic Esophagitis [p27/table2]
- Sanofi and Regeneron Data on File. 2.7.4 Summary of Clinical Safety (PN) dupilumab (SAR231893/REGN668) [p47/table11, p63/table19]