BACKGROUND – Allergic rhinitis and air pollution
- Allergic Rhinitis (AR) is a multifactorial disease, with some evidence supporting a correlation between air pollutants and AR1
- A recent Lancet Commission suggests that pollution is the largest environmental cause of death and disease globally, with the burning of combustible fuel and biomass accounting for around 85% of airborne particulate pollution2
- Diesel exhaust particles (DEP) have been estimated to account for up to 80% of human exposure to particulate matter (PM) and most airborne PM in the world’s largest cities3
- Epidemiological studies have shown a relationship between air pollutants and an increase in allergic/respiratory symptoms4
- Individuals with AR may develop nasal hyper-responsiveness from various stimuli, rendering them more responsive to airborne irritants - likely to be involved in exacerbating symptoms of AR4
- London NR et al. World J Otorhinolaryngol Head Neck Surg 2018;4:209-15.
- Landrigan PJ et al. Lancet 2018;391:462-512.
- Huang S-K et al. J Thorac Dis 2015;7:23-33.
- Dunlop J et al. Immunol Allergy Clin North Am 2016;36:367-77.
FEXPOLSAR Study: Schematic of Study Design
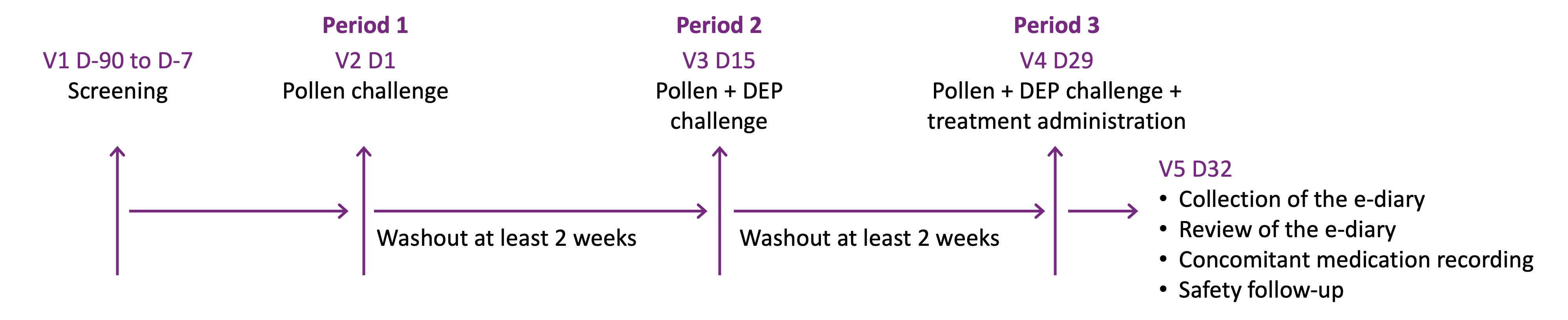
Screening:
- Eligibility criteria
- Informed consent
- Skin prick test
- Blood sampling
- Vital signs
- Physical Exam
- Nasal Exam
Period 1:
- H0 to H+3: Challenge to pollen (266 subjects)
- H0 to H+3: Symptoms evaluation every 30min (TNSS ≥ 3 verification)
- H+3: Discharge from EEU
- H+3 to H+12: At home, symptoms evaluation every 60 min
- H+12: End of symptoms evaluation; AE evaluation and concomitant medication recording
Period 2:
- H0 to H+3: Challenge to pollen + DEP (261 subjects)
- H0 to H+3: Symptoms evaluation every 30min
- H+3: Discharge from EEU H+3 to H+12: At home, symptoms evaluation every 60 min
- H+12: End of symptoms evaluation; AE evaluation and concomitant medication recording
Period 3:
- H0 to H+3: Challenge to pollen + DEP (253 subjects)
- H+2: Randomization and IMP administration
- H+3: Subjects discharge from EEU
- H+3 to H+12: At home, symptoms evaluation every 60 min
- H+12: End of symptoms evaluation; AE evaluation and concomitant medication recording
Adapted from Ellis A K et al. WAO J, 2020, abstract in press; Ellis AK et al, EAACI 2020
AE=adverse event, D=day, DEP=diesel exhaust particles, EEU=environmental exposure unit, H=hour, IMP=investigational medical product, mITT=modified intent-to-treat, TNSS=total nasal symptom score, V=visit. Note: V5 D32 was allowed to be attended by a phone call
FEXPOLSAR Study: Schematic
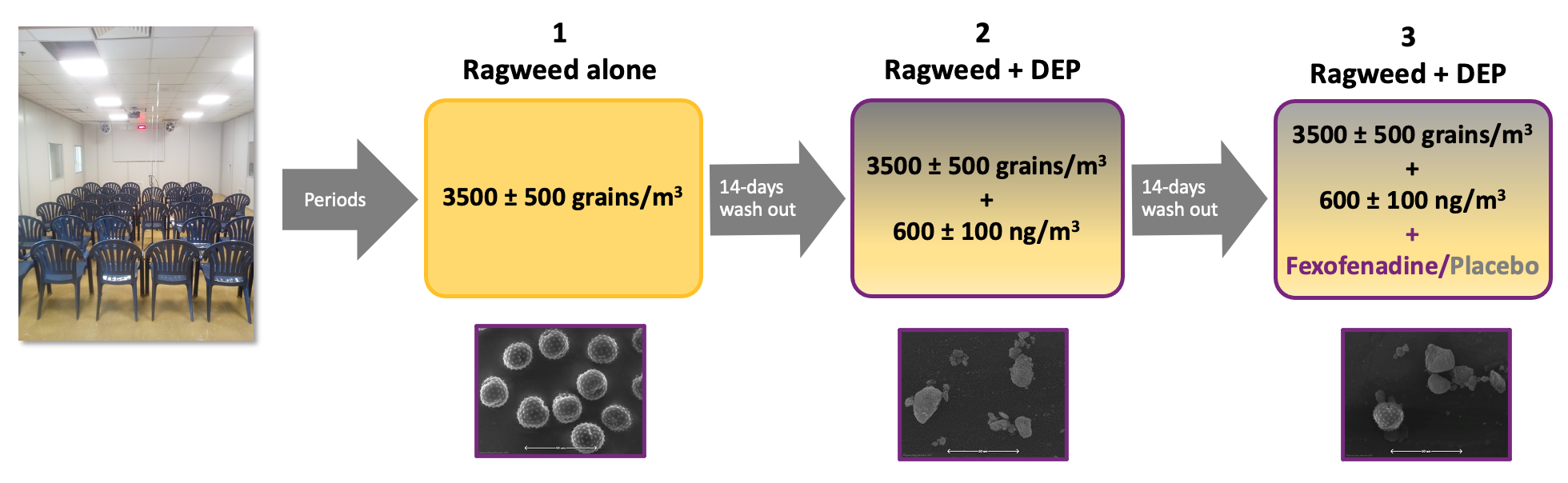
Transmission electron microscope images of ragweed pollen and DEP at 1000x magnification
Adapted from Ellis A K et al. WAO J, 2020, abstract in press; Ellis AK et al, EAACI 2020
DEP=diesel exhaust particles
FEXPOLSAR Study: Subject disposition
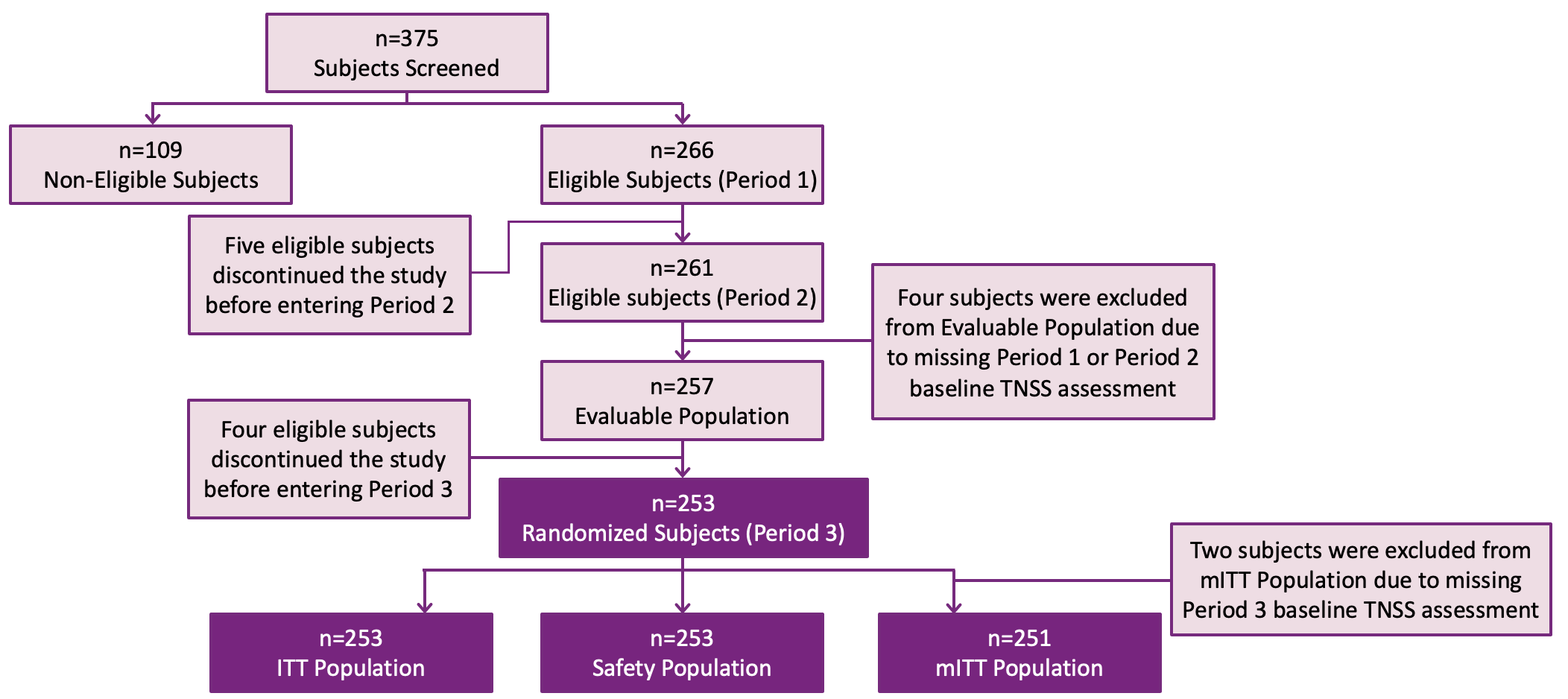
Adapted from Ellis A K et al. WAO J, 2020, abstract in press; Ellis AK et al, EAACI 2020
ITT=intent-to-treat, mITT=modified intent-to-treat, TNSS=total nasal symptom score
FEXPOLSAR STUDY: SUBJECT DEMOGRAPHICS AT BASELINE
| Evaluable population | mITT population | |||
| Demographics | All N=257 |
Placebo N=125 |
Fexofenadine HCI 180 mg N=126 |
All N=251 |
| Mean age, years (SD) | 40·8 (12·6) | 41·5 (12·5) | 40·0 (12·6) | 40·7 (12·5) |
| Sex, n (%) | ||||
| Male | 90 (35·0) | 37 (29·6) | 49 (38·9) | 86 (34·3) |
| Female | 167 (65·0) | 88 (70·4) | 77 (61·1) | 165 (65·7) |
| Smoking Status | ||||
| Never smoked | 173 (67·3) | 77 (61·6) | 92 (73·0) | 169 (67·3) |
| Quit smoking | 52 (20·2) | 30 (24·0) | 22 (17·5) | 52 (20·7) |
| Currently smokes | 32 (12·5) | 18 (14·4) | 12 (9·5) | 30 (12·0) |
| Allergic medical history, n (%) | ||||
| Seasonal Allergy | 257 (100·0) | 125 (100·0) | 126 (100·0) | 251 (100·0) |
| Perennial Rhinitis | 183 (71·2) | 85 (68·0) | 92 (73·0) | 177 (70·5) |
Adapted from Ellis A K et al. WAO J, 2020, abstract in press; Ellis AK et al, EAACI 2020
Ellis A K et al. WAO J, 2020, abstract in press; Ellis AK et al, EAACI 2020
FEXPOLSAR STUDY: Mean + SE TNSS for AUC from H0 to H12 in Periods 1 and 2 (Evaluable Population)

*p-value was obtained using a mixed model for repeated measures (MMRM) on log transformed values of TNSS AUC (H0-H12) plus 0.1, adjusted on baseline TNSS (H0) for each period (1 and 2) and on pollen counts at each EEU session, with period as a fixed categorical effect.
AUC=area under the curve, DEP=diesel exhaust particles, H=hour, SE=standard error , TNSS=total nasal symptom score
FEXPOLSAR STUDY: Mean TNSS + SE from H+2 to H+12
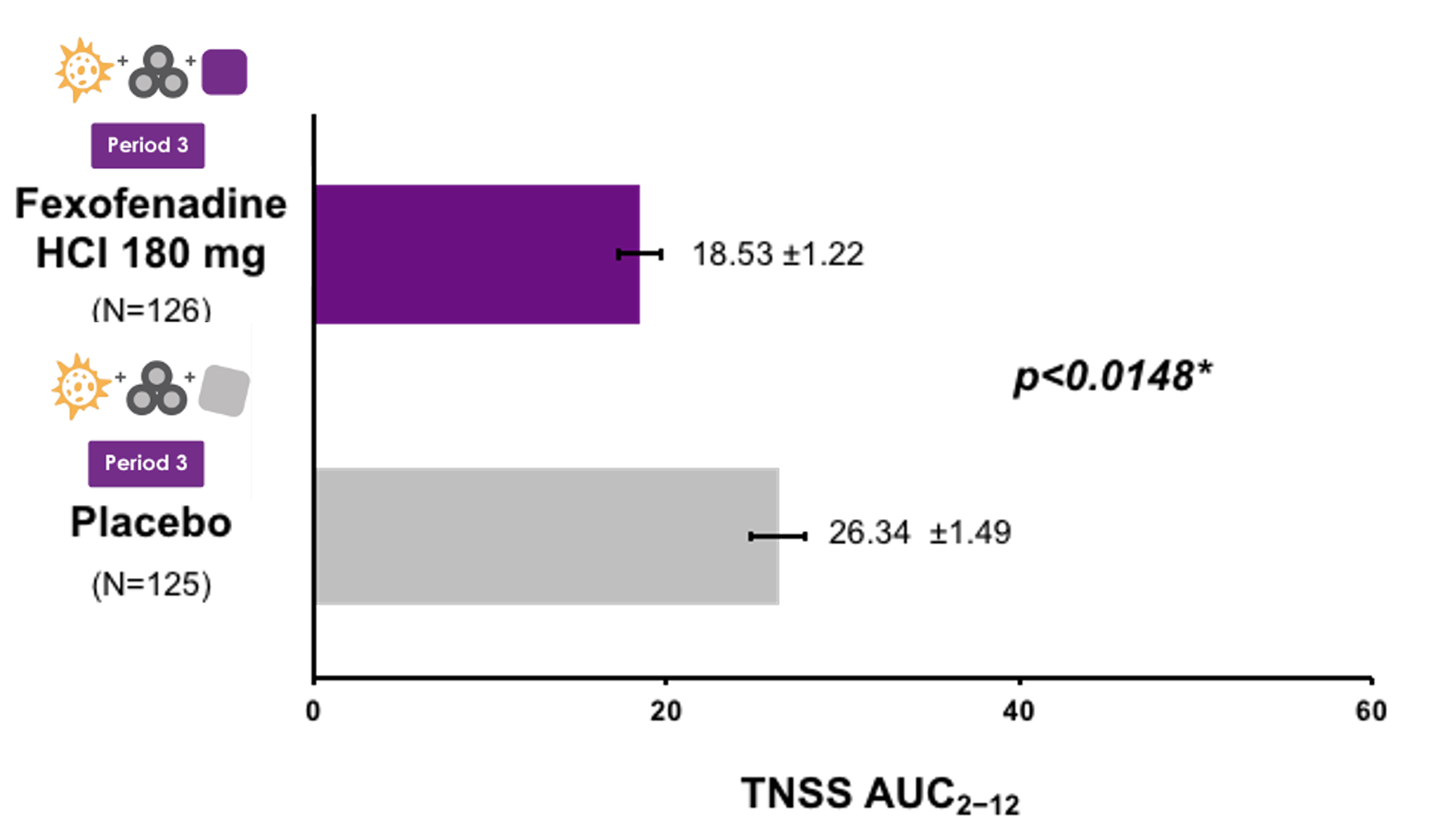
*p: Analysis of covariance (ANCOVA) of Log transformed values of TNSS AUC2-12 plus 0.1, with treatment group as a fixed categorical effect and baseline TNSS (H+12) as covariate
AUC=area under the curve, H=hour, HCl=hydrochloride; mITT=modified intent-to-treat, SE=standard error, TNSS=total nasal symptom score
FEXPOLSAR STUDY: INDIVIDUAL SYMPTOM SCORES
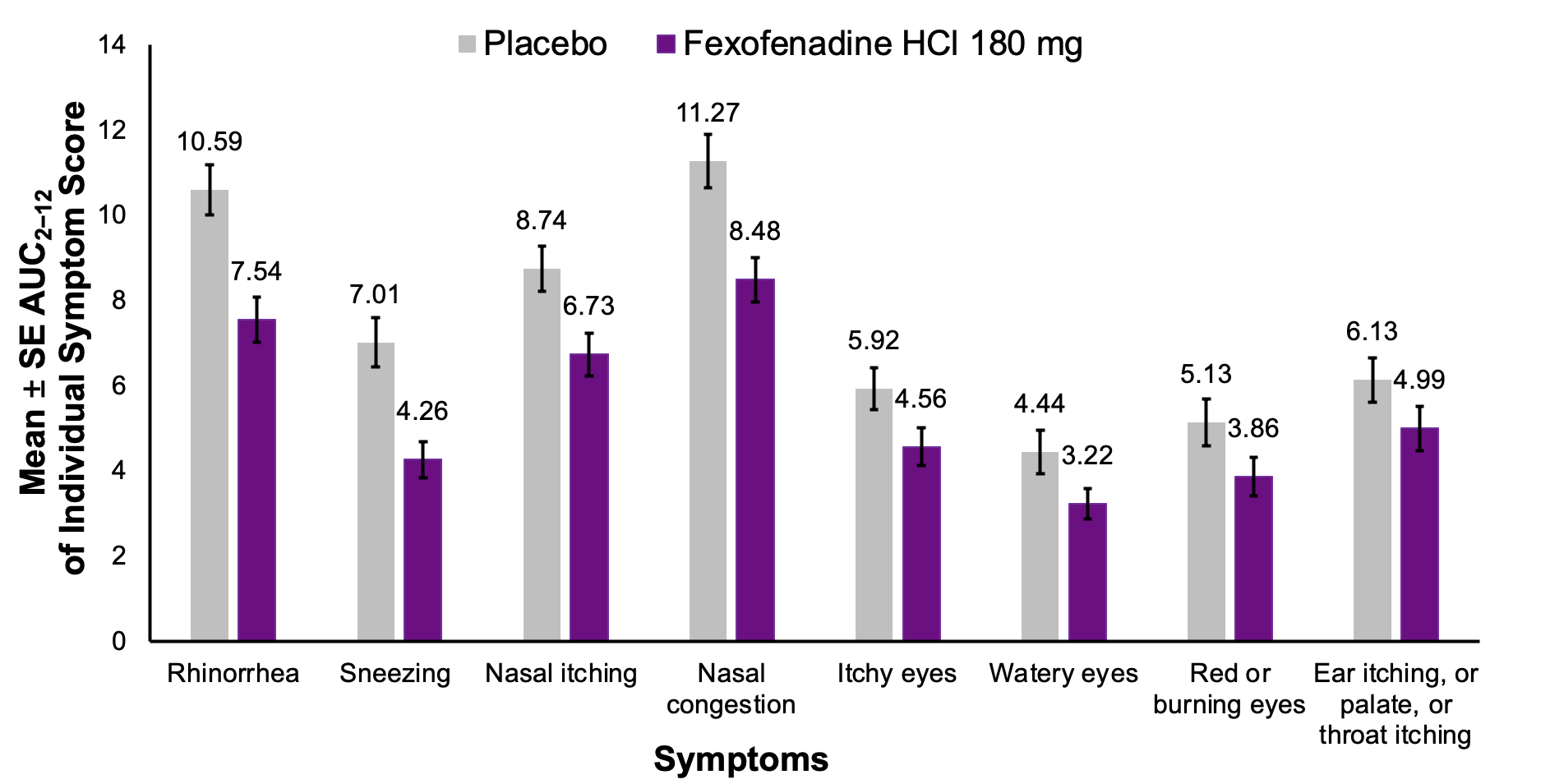
*Secondary endpoint; Foot note: DEP= diesel exhaust particles, mITT= modified intent-to-treat; SE, standard error
- Ellis A K et al. WAO J, 2020, abstract in press; Ellis AK et al, EAACI 2020
FEXPOLSAR STUDY: ADVERSE EVENTS OCCURRING IN ≥2% OF PATIENTS IN EITHER TREATMENT GROUP
| Primary system organ class (prefferred term) | Placebo, n (%) N=126 |
Fexofenadine HCI, n (%) N=127 |
| Any class | 19 (15·1) | 16 (12·6) |
| Immune system disorders | 7 (5·6) | 6 (4·7) |
| Seasonal allergy | 7 (5·6) | 6 (4·7) |
| Respiratory, thoracic and mediastinal disorders | 5 (4·0) | 2 (1·6) |
| Infections and infestations | 3 (2·4) | 2 (1·6) |
| Gastrointestinal disorders | 0 (0·0) | 3 (2·4) |
- Ellis A K et al. WAO J, 2020, abstract in press; Ellis AK et al, EAACI 2020
FEXPOLSAR STUDY - CONCLUSIONS
- Among adults with documented ragweed allergy, controlled exposure to DEP + ragweed pollen in the EEU significantly increased SAR symptoms compared to ragweed exposure alone
- DEP rapidly aggravated pollen-induced SAR symptoms and this effect persisted beyond the end of the DEP + pollen exposure period
- Fexofenadine HCl 180 mg significantly reduced these pollution-aggravated symptoms, compared with placebo
- Based on these data, we conclude that
- Air pollutants can significantly exacerbate SAR symptoms
- and that
- Fexofenadine HCl 180 mg is an effective and well-tolerated treatment that has been shown to significantly alleviate these pollution-aggravated symptoms
References: Telfast® 180mg film-coated tablets Patient Information leaflet; Revision date January 2019
Disclaimer: *Fexofenadine HCI 180 mg is registered in both markets of UAE and KSA please refer to the respective Patient Information leaflet in the reference tab
Sanofi-aventis Australia pty ltd 12-24 Talavera Road Macquarie Park NSW 2113 Australia.
Consumer HealthCare Details:
SAUDI ARABIA:
SANOFI, Kingdom of Saudi Arabia, P.O. Box 9874, Jeddah 21423, K.S.A. Tel: +966-12-669-3318, Fax: +966-12-663-6191
For Pharmacovigilance, Please contact: +966-54-428-4797, ksa_pharmacovigilance@sanofi.com
To report any side effect(s): The National Pharmacovigilance and Drug Safety Centre (NPC) • Fax: +966-11-205-7662 SFDA call center: 19999 • E-mail: npc.drug@sfda.gov.sa • Website: https://ade.sfda.gov.sa/
To report any Product Technical Complaint, please contact SANOFI Quality Department: Email: quality.greatergulf@sanofi.com
MAT-SA-2300120/V1/ March 2023
