Loss of Smell Improvement in Patients With CRSwNP
Loss of Smell Improvement in Patients With CRSwNP:
Results From SINUS-24 and SINUS-52a
These materials are presented by Sanofi and Regeneron Medical for scientific exchange purposes only. Please refer to the product label for full prescribing information. Figures were reproduced with permission from the publisher.
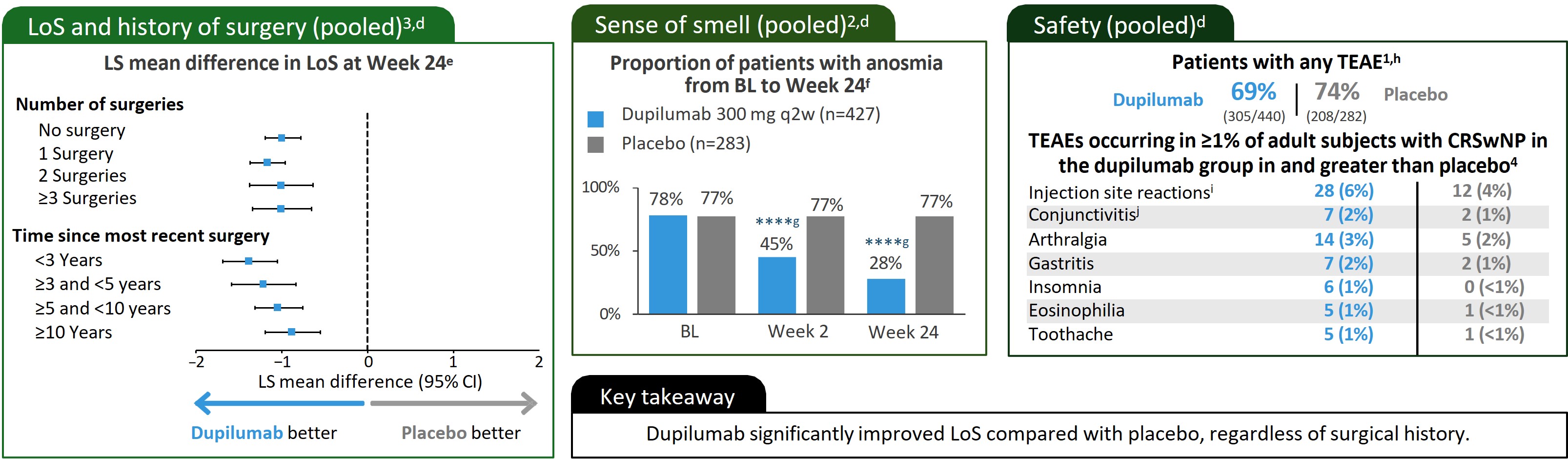
*P<0.05/**P<0.01/***P<0.001 /****Nominal P<0.0001 vs placebo.
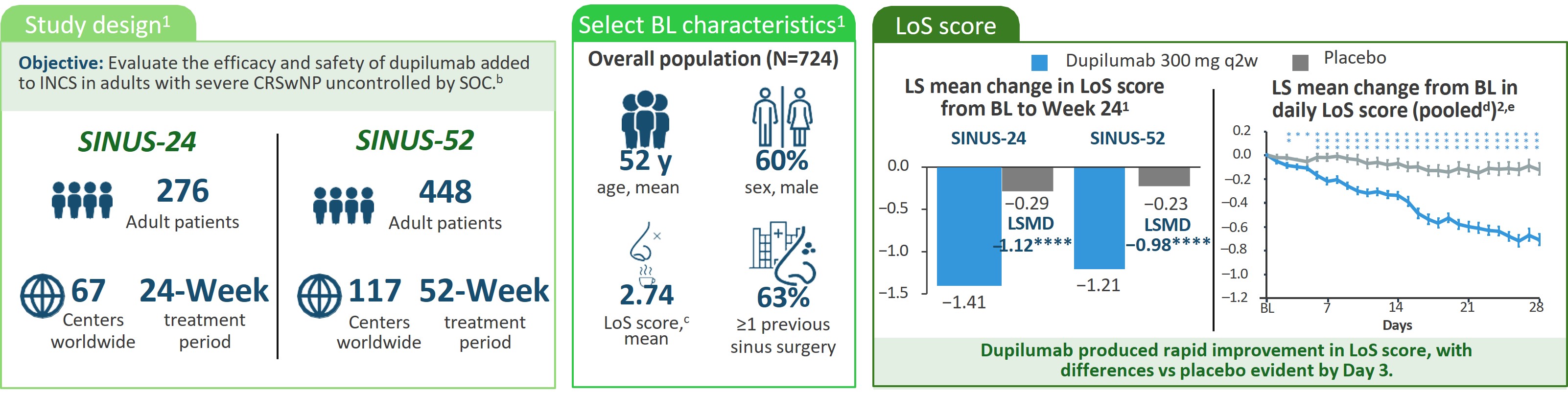
aPlease see manuscripts for full details.1–3 bEligible patients were aged ≥18 years with bilateral nasal polyps and symptoms of CRS despite INCS therapy before randomization and had received SCS in the preceding 2 years (or had a medical contraindication or intolerance to SCS) or previous sinonasal surgery. In SINUS-24, patients were randomly assigned (1:1) to dupilumab 300 mg SC q2w or to matching placebo. In SINUS-52, patients were randomly assigned (1:1:1) to dupilumab 300 mg SC q2w for 52 weeks, the same schedule for the first 24 weeks followed by dupilumab 300 mg SC q4w, or placebo. cScale 0–3: Higher scores indicate greater disease severity. dPooled data from SINUS-24 and SINUS-52. e(n=724). fOnly the 710 patients who completed an UPSIT assessment at BL were included in this analysis. gNominal.hTwo deaths occurred during the study period that were deemed unrelated to the study drug; 1 patient given placebo in SINUS-24 had suspected myocardial infarction occurring after the period of TEAEs, and 1 treated with dupilumab in SINUS-52 had intracranial hemorrhage after a fall, occurring within the period of TEAEs. These patients were not included in the safety population. iInjection site reactions cluster included injection site reaction, pain, bruising and swelling. jConjunctivitis cluster included conjunctivitis, allergic conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, giant papillary conjunctivitis, eye irritation, and eye inflammation.
BL, baseline; CRS, chronic rhinosinusitis; INCS, intranasal corticosteroids; LoS, loss of smell; LS, least-squares; LSMD, least-squares mean difference; q2w, every 2 weeks; q4w, every 4 weeks; SC, subcutaneously; SCS, systemic corticosteroids; SOC, standard of care; TEAE, treatment-emergent adverse event; UPSIT, University of Pennsylvania Smell Identification Test.
1. Bachert C, et al. Lancet. 2019;394:1638–1650. 2. Mullol J, et al. J Allergy Clin Immunol Pract. 2022;10:1086-1095.e5. 3. Hopkins C, et al. Int Forum Allergy Rhinol. 2021;11:1087–1101. 4. Dupixent (dupilumab) Prescribing Information. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; Tarrytown, NY: Regeneron Pharmaceuticals, Inc. 2024
Loss of Smell Improvement in Patients With CRSwNP:
Real-World Dataaa
These materials are presented by Sanofi and Regeneron Medical for scientific exchange purposes only. Please refer to the product label for full prescribing information.
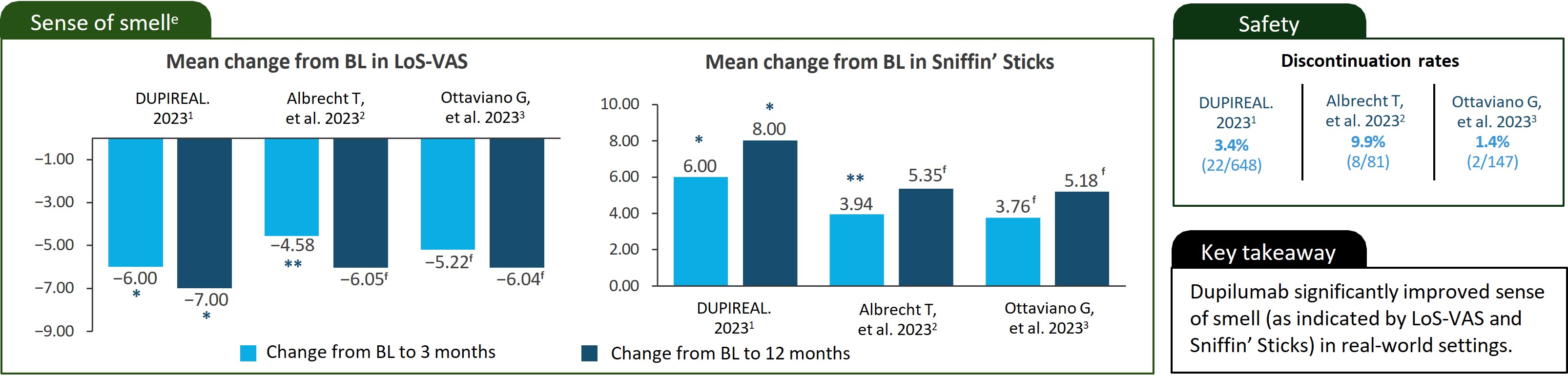
*P<0.001/ **P<0.0001.
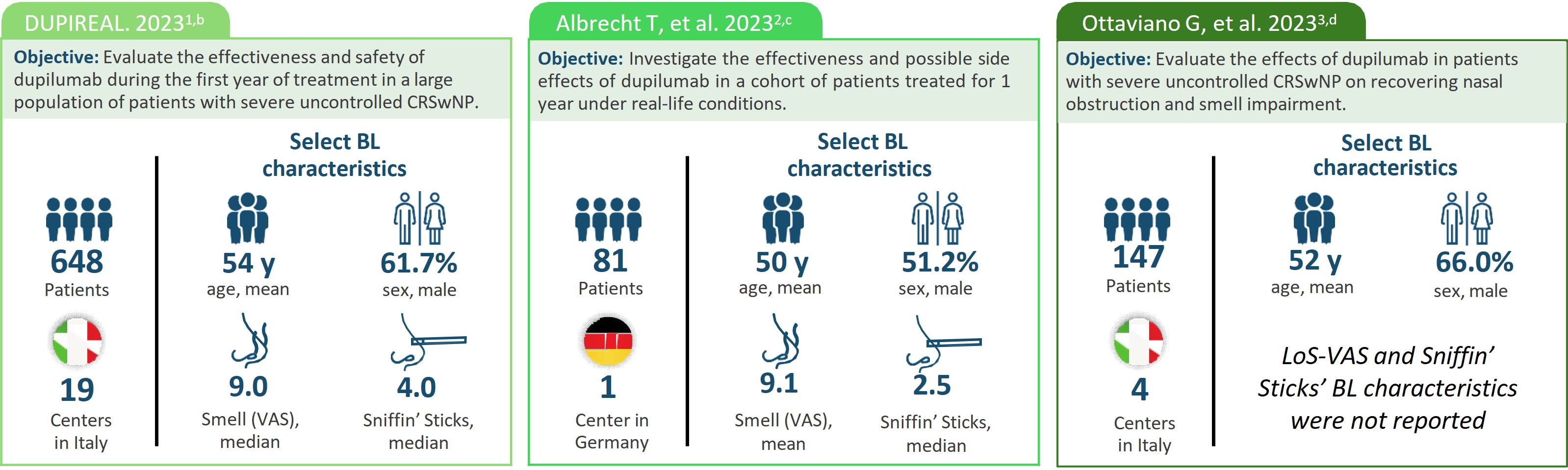
aPlease see manuscripts for full details.1–3 Note that real-world data are collected from diverse sources outside the controlled environment of a clinical trial. As such, the reliability of real-world data can be hindered by data quality issues, confounding variables, and limitations in establishing causality.4 bA phase 4 real-life, observational, retrospective, multicenter study that assessed the effectiveness and safety of dupilumab 300 mg SC q2w in patients with severe uncontrolled CRSwNP over the first year of treatment. Patients enrolled were treated between November 2020 and March 2022. cA real-world prospective observational study that included patients with CRswNP who underwent treatment with dupilumab. Patients were treated with dupilumab 300 mg SC q2w as an add-on therapy to INCS. The treatment period for this study wasnot specified in the manuscript. dAn observational, retrospective multicenter study. Patients were treated with dupilumab 300 mg SC q2w as an add-on therapy to INCS for ≥1 year and followed between February 2020 and November 2022. eData from different studies are not meant to be directly compared. Data reported are not comprehensive. fThe manuscript does not provide data on the statistical significance of the change from baseline to the specified timepoint.
BL, baseline; INCS, intranasal corticosteroids; LoS, loss of smell; q2w, every 2 weeks; SC, subcutaneously; VAS, Visual Analogue Scale.
1. De Corso E, et al. Allergy. 2023;78:2669–2683. 2. Albrecht T, et al. World Allergy Organ J. 2023;16:100780. 3. Ottaviano G, et al. J Pers Med. 2023;13:234. 4. Kim HS, Kim JH. J Korean Med Sci. 2019;34(4):e28.
