- Article
- Source: Campus Sanofi
- 23 Oct 2023
Indication and Mechanism of action of Clexane (enoxaparin)


Indication
The prophylaxis of venous thromboembolism (VTE):1
- In moderate and high risk surgical patients, in particular those undergoing orthopaedic or general surgery including cancer surgery.
- In medical patients with an acute illness and reduced mobility at increased risk of VTE.
The treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), excluding PE likely to require thrombolytic therapy or surgery:1
- Extended treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) and prevention of its recurrence in patients with active cancer.
- Prevention of thrombus formation in extracorporeal circulation during haemodialysis.
- Acute coronary syndrome:
- Treatment of unstable angina and Non ST-segment elevation myocardial infarction (NSTEMI), in combination with oral acetylsalicylic acid.
- Treatment of acute ST-segment elevation myocardial infarction (STEMI) including patients to be managed medically or with subsequent percutaneous coronary intervention (PCI).
Mechanism of action
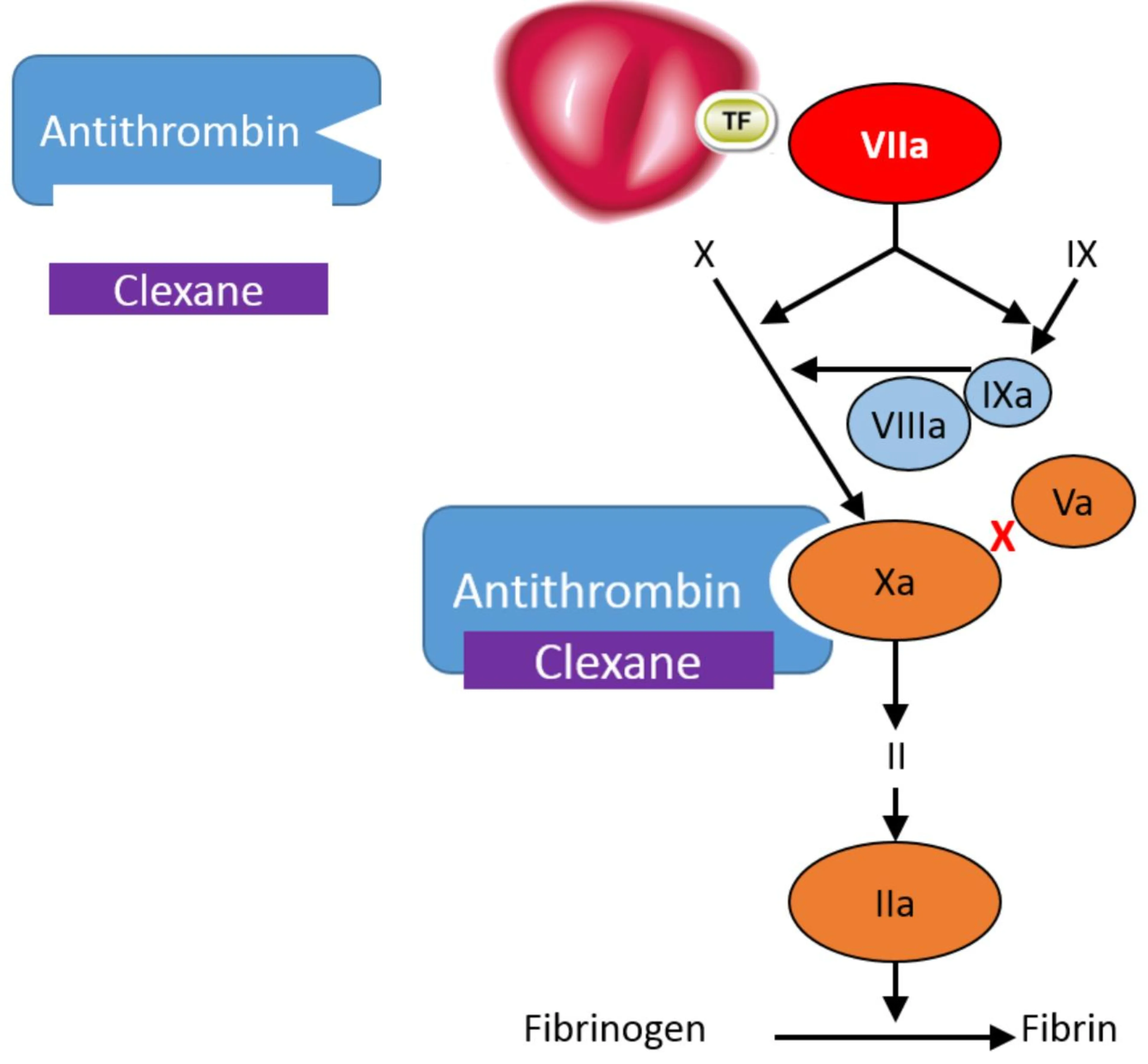
Clexane® exerts its anticoagulant effect by preventing the formation of blood clots through binding to antithrombin, a naturally occurring coagulation inhibitor and potentiating its action.2
Antithrombin is a natural inhibitor of the coagulation factors FXIa, FIXa, FXa and FIIa (thrombin).2
Clexane® forms a complex with antithrombin. This complex undergoes a conformational change; in its altered conformation, the complex inhibits FXa, which is the primary mechanism of action. It also inhibits FIIa, although to a lesser extent - in vitro, Clexane has a high anti-Xa activity and low anti-IIa or anti thrombin activity, with a ratio of 3.6 :1.2
.png)
References
- Clexane Pre-filled Syringes Summary of Product Characteristics. February 2022. Please consult the SmPC for full information.
- Carter NJ, McCormack PL, Plosker GL (2008). Enoxaparin: a review of its use in ST-segment elevation myocardial infarction. Drugs, 68:691–710.
MAT-XU-2204403 (v2.0) Date of Preparation: October 2023