- Article
- Source: Campus Sanofi
- 23 Oct 2023
Recombinant Flu Vaccine
.jpg)
This is intended for HCPs practising in Great Britain (England, Scotland and Wales) only.
Supemtek®▼(Quadrivalent recombinant Influenza Vaccine, prepared in cell-culture) Prescribing Information
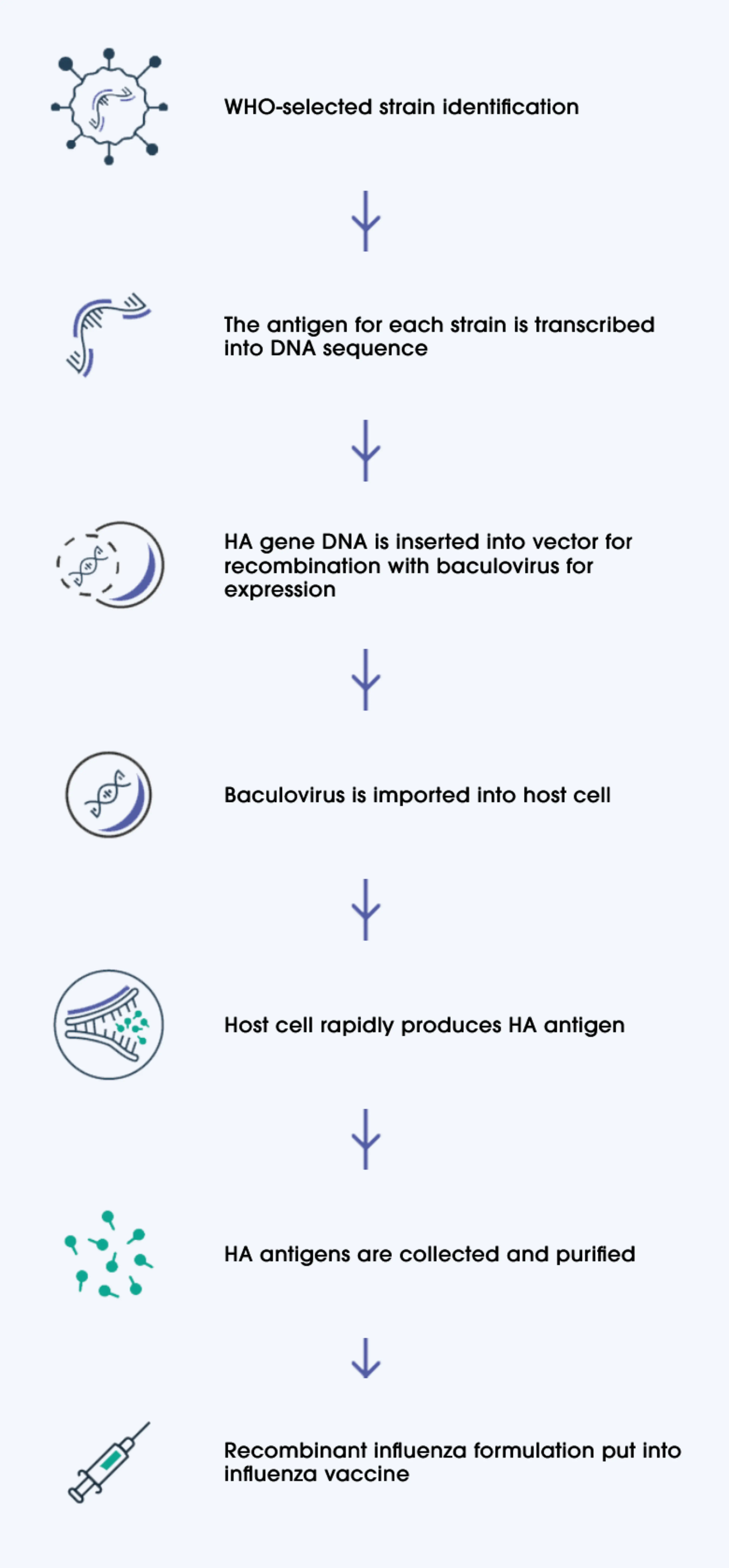
How Supemtek is manufactured
Recombinant technology eliminates the possibility of adaptation or mutation by replicating only the haemagglutinin (HA) antigen, directly from a genetic sequence. The resulting antigens are an exact genetic match to the target strain.2,3
In the production of Supemtek, the HA antigen DNA for each World Health Organization (WHO)-recommended influenza strain is combined with host cell DNA.1,3 Because only the DNA for each influenza antigen is used, there is no live virus replication during the recombinant process.3
Supemtek contains 3x more HA antigen (45 μg versus 15 μg) per strain, compared with cell- and egg-based standard-dose quadrivalent influenza vaccines.2
Discover more about how Supemtek, our recombinant flu vaccine, is manufactured:
Recombinant technology generates HA antigens that are an exact genetic match to the target strains2,3
This eliminates the possibility of adaptation or mutation.2,3
According to evidence gathered over previous flu seasons, influenza vaccine effectiveness against A/H3N2 influenza viruses may have been lower than against other influenza strains. This may be due to egg adaptation, resulting in an antigenic mismatch between the vaccine strain and strains circulating during a particular influenza season.5–8
CVV, Candidate Vaccine Virus; HA, haemagglutinin; WHO, World Health Organization.
References
- Supemtek Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/12761/smpc#gref. (Accessed October 2023).
- Dunkle LM, et al. N Engl J Med. 2017;376:2427–36.
- Centers for Disease Control and Prevention (CDC). How influenza (flu) vaccines are made. https://www.cdc.gov/flu/prevent/how-fluvaccine-made.htm#recombinant (Accessed October 2023).
- Harding AT and Heaton NS. Vaccines. 2018;6:19.
- Skowronski DM, et al. PLoS ONE. 2014;9:e92153
- Zost SJ, et al. Proc Natl Acad Sci USA. 2017;114:12578–83.
- Widjaja L, et al. Virology. 2006;350:137–45.
- Flannery B, et al. Morb Mortal Wkly Rep. 2018;67:180–5.
MAT-XU-2301386 (v2.0) Date of preparation: December 2023