Proven Efficacy of Dupixent® against Type 2 Inflammation

Mechanism of Action
Dupixent has emerged as a groundbreaking therapeutic option for various type 2 inflammatory diseases. As a fully human monoclonal antibody, Dupixent uniquely inhibits the signaling of both interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways, key drivers of type 2 inflammation 1,2.
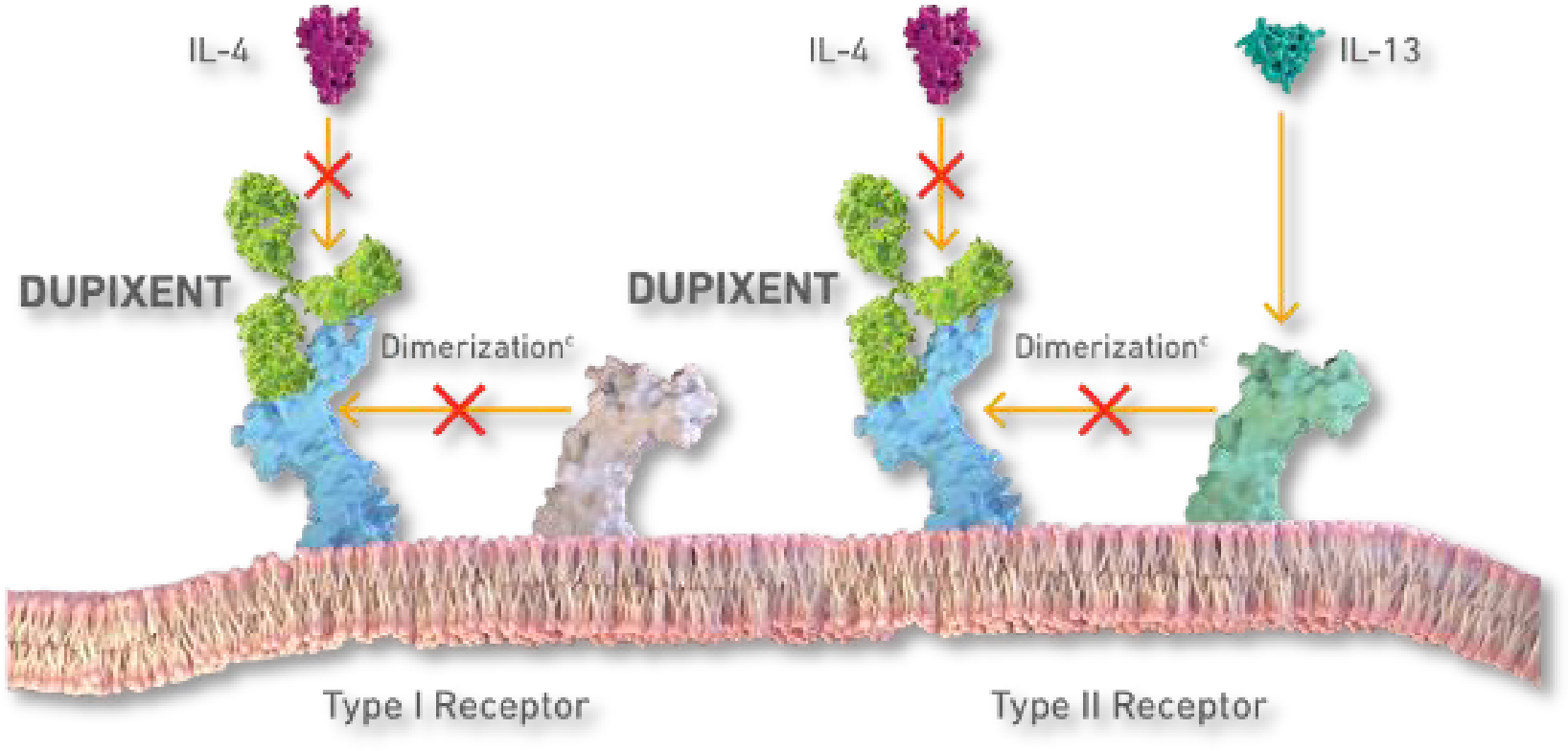
Figure 1: DUPIXENT is the only dual inhibitor of IL-4 and IL-13 signaling3
This mechanism of action has demonstrated significant clinical benefits across multiple indications, including Atopic Dermatitis, Asthma, Chronic Rhinosinusitis with Nasal Polyposis, Eosinophilic Esophagitis, Prurigo Nodularis, Chronic Spontaneous Urticaria, and most recently, Chronic Obstructive Pulmonary Disease2 with regulatory approvals in >60 countries and over 1,000,000 patients treated globally1.
Dupixent has rapidly become an essential therapy in the management of type 2 inflammatory diseases and has demonstrated sustained improvement in symptoms across various indications, with long-term safety data available for up to 5 years in some conditions 1,2.
This unique mechanism of action has demonstrated significant clinical benefits across multiple type 2 inflammatory diseases as seen in the 3 examples below:
Atopic Dermatitis (AD):
1.Mechanism of Action:
Targeting of IL-4 and IL-13 signaling, key drivers of type 2 inflammation in AD4

2.Long-term Efficacy:
39%
Achieved IGA score of 0-1 (clear/ almost clear) with a reduction of ≥2 points on a 0-4 IGA scale at 16 weeks in adults®
42.7%
Had an IGA score of 0/1 (clear/almost clear) by week 52 in adolescents (12-17 years)5
Sustained improvement in skin clearance up to 52 weeks in adults®
3. Real-World Evidence:
5-year clinical trial data and experience in > 1,000,000
patients worldwide across all indications have proven Dupixent's efficacy*

Approved for use in patients as young as 6 months old5
Asthma

1.Mechanism of Action:
Targeting of IL-4 and IL-13 signaling, reducing Type 2 Inflammation in airways4,6

2.Long-term Efficacy:
Up to 3 years of data show sustained improvment in lung function and reduction in severe exacerbations4,7
13-22%
Improvement in lung function (FEV-1) at 96 weeks 4

3. Real-World Evidence:
> 2,200 patients
Enrolled in the Phase 3 open-label extension trial making it the largest biologic medicine trial in Asthma4,8
Chronic Obstructive Pulmonary Disease (COPD):

1.Mechanism of Action:
Targeting of IL-4 and IL-13
pathways to reduce Type 2 Inflammation in COPD7

2.Long-term Efficacy:
30% and 34%
Annualized rate of moderate or severe COPD exacerbations over 52 weeks in BOREAS and NOTUS trial7

Improvements in lung function (FEV-1) observed as early as week 2 and sustained at 52 weeks7

Enhanced health-related quality of life7

3. Real-World Evidence:
1st
new treatment approach for COPD in more than a decade7

Approved for ~220,000 adults in the European Union7
In Conclusion
Dupixent (dupilumab) has proven to be a transformative treatment option for multiple type 2 inflammatory diseases by targeting IL-4 and IL-13 pathways.
Its efficacy across diverse indications demonstrates significant improvement in patient outcomes, reducing exacerbation rates, enhancing lung function, and improving skin clarity and quality of life.
Long-term data highlight its consistent safety profile across various age groups, further supported by real-world evidence.
Dupixent remains a promising therapy for sustained disease control and relief from the burdens of type 2 inflammation.
- https://www.sanofi.com/en/media-room/press-releases/2024/2024-02-23-06-00-00-2834219
- https://www.dupixenthcp.com/prurigo-nodularis/about/mechanism-of-action
- https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-11-05-05-00-2944239
- https://www.sanofi.com/en/media-room/press-releases/2024/2024-05-31-05-00-00-2891259
- Regeneron Pharmaceuticals. CHRONOS CTR report full body. [p190/table50, p201/table56, p205/table57)
- Castro, M., et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma New England Journal of Medicine. (2018). [p8/table2, p9/col1-col2/h1-h3]
- Bachart C, Han JK, Desrosiers M, et al. (2019) "Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials". Lancet. 2019 Sep 19. (Table S12, Table S13)
- Dellon E., et al 2022. Dupilumab in Adult and Adolescent Patients with Eosinophilic Esophagitis [p27/table2]
- Sanofi and Regeneron Data on File. 2.7.4 Summary of Clinical Safety (PN) dupilumab (SAR231893/REGN668) [p47/table11, p63/table19]
