ISTH Guidelines for the Diagnosis and Management of aTTP (iTTPa)

Overview of Guidelines
published by ISTH
and patient representatives
diagnosis and treatment of TTP
or hereditary (congenital) TTP
Need for Treatment Guidelines
Despite recent advances in diagnosis and treatment, TTP remains a serious challenge to HCPs and patients1
- TTP is a life-threatening blood disorder with substantial mortality and morbidity in the acute phase. Due to the rare nature of the disease, many HCPs have limited experience with managing TTP
- Evidence on optimizing early diagnosis and management of acute and recurrent episodes is limited
- There is large variation in clinical practice among experts for disease management
- Two guidance documents have been published for the diagnosis and management of TTP to date, by the following groups
- British Committee for Standards in Haematology2
- Japan’s Blood Coagulation Abnormalities Research Team3
- There have since been significant advances in the diagnosis and treatment of TTP; more published data are now available on impact of diagnostic strategies on objective health outcomes
HCP, healthcare provider; ISTH, International Society on Thrombosis and Haemostasis; TTP, thrombotic thrombocytopenic purpura
- Zheng XL, et al. J Thromb Haemost. 17 July 2020; doi:10.1111/JTH.15006.
- Scully M, et al. Br J Haematol. 2012;158(3):323-335.
- Matsumoto M, et al. Int J Hematol. 2017;106(1):3-15.
Guideline Development
Multidisciplinary panel1,2
- Clinical experts in the diagnosis and management of aTTP (including hematologists and pathologists) and physicians in other relevant disciplines
- Patient representatives
Literature review
- A search of electronic databases MEDLINE, Embase, and the Cochrane Library (treatment) or MEDLINE and Embase up to February 5, 2019 (diagnosis)1,2
- Included RCTs and observational studies (including registry data)1
- Population of interest: platelet counts <150 × 109/L, anemia, preserved renal function, and schistocytes2
- Subjects must have been receiving ≥1 treatment of interest: TPE with/without adjuvant corticosteroids, rituximab, or caplacizumab2
Scope
- Diagnosis and treatment of adults with suspected or diagnosed TTP (acquired and congenital) during acute events and while in remission1,2
- Risk assessment models (ie, PLASMIC and French scores) were considered out of scope2
aTTP, acquired thrombotic thrombocytopenic purpura; iTTP, immune-mediated thrombotic thrombocytopenic purpura. RCT, randomized controlled trial; TPE, therapeutic plasma exchange; TTP, thrombotic thrombocytopenic purpura.
- Zheng XL, et al. J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010. 2. Zheng XL, et al. J Thromb Haemost. 17 July 2020; doi:10.1111/JTH.15006.
PICO Framework for Treatment Questions
For patients experiencing a first acute event or a relapse event of aTTP (iTTPa)2
- Should TPE plus corticosteroids versus TPE alone be used?
- Should rituximab be added or not to TPE and corticosteroids?
- Should caplacizumab be used or not?
For patients with aTTP (iTTP) or cTTP in remission2
- Should prophylactic rituximab therapy be used or not?
- Should plasma infusion versus a watch-and-wait strategy be used?
- Should a FVIII concentrate infusion versus a watch-and-wait strategy be used?
- Should plasma infusion versus a FVIII concentrate infusion be used?
For patients with aTTP (iTTP) or cTTP in remission during pregnancy2
- Should prophylactic immunosuppression versus a watch-and-wait strategy be used for patients with a decreased level of plasma ADAMTS13 but without other signs/symptoms of TMA?
- Should plasma infusion versus a FVIII concentrate infusion be used?
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aTTP, acquired thrombotic thrombocytopenic purpura; cTTP, congenital thrombotic thrombocytopenic purpura; FVIII, factor VIII; PICO, population, intervention, comparison, outcome; iTTP, immune-mediated thrombotic thrombocytopenic purpura; TMA, thrombotic microangiopathy; TPE, therapeutic plasma exchange; TTP, thrombotic thrombocytopenic purpura.
aNote that aTTP and iTTP are synonyms.
- Zheng XL, et al. J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010. 2. Zheng XL, et al. Suppl J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010.
GRADE Approach to Summarize Evidence
GRADE approach: focus on outcomes “important” for decision-making, defined as outcomes that are relevant to patients, measurable, and clearly defined
- Assessed the certainty of the evidence based on three scenarios
- Where ADAMTS13 activity measurement is readily available (ie, within 72 hours)
- Where ADAMTS13 measurement is NOT available
- Where ADAMTS13 activity measurement is available with a delay (ie, after 72 hours, but <7 days)
Strong recommendation
- Usually based on high-quality evidence in which experts have high confidence
- The panel is confident that the desirable effects outweigh the undesirable effects
- Most patients would accept the recommended course of action
- Most clinicians should follow the recommended course of action, which can be largely adopted as a policy
Conditional recommendation
- The panel believes that the desirable effects probably outweigh the undesirable effects
- Most patients would accept the suggested course of action, but many may not
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aTTP, acquired thrombotic thrombocytopenic purpura; GRADE, Grading of Recommendations Assessment, Development and Evaluation; RCT, randomized controlled trial
- Zheng XL, et al. J Thromb Haemost. 17 July 2020; doi:10.1111/JTH.15006.
Population of Interest
Diagnostic pathways apply to patients presenting with clinical thrombotic microangiopathy and suspected TTP
- Patient populations of interest:
- Thrombocytopenia (<150 ×109/L)
- Microangiopathic hemolytic anemia
- Hemoglobin and hematocrit < LLN
- Low haptoglobin
- Elevated lactate dehydrogenase
- Schistocytes present in blood smear
- Relatively preserved renal function
LLN, lower limit of normal; TTP, thrombotic thrombocytopenic purpura.
- Zheng XL, et al. J Thromb Haemost. 17 July 2020; doi:10.1111/JTH.15006.
Summary of Diagnostic Recommendations
| Recommendation number | Clinical suspicion of TTP | Key recommendations | Strength of recommendation | Evidence grade |
|---|---|---|---|---|
| Timely access to ADAMTS13 | ||||
| 1 | High | • Start TPE and corticosteroids before receiving ADAMTS13 test results • Consider starting caplacizumab early before receiving ADAMTS13 test results • Adjust therapies based on ADAMTS13 results (details in following slides) | Conditional | Low certainty |
| 2 | Low certainty | • Consider starting TPE and corticosteroids before receiving ADAMTS13 test resultsa • Do NOT start caplacizumab until ADAMTS13 test results are received • Adjust therapies based on ADAMTS13 results (details in following slides) | Conditional | Low certainty |
| ADAMTS13 testing not available | ||||
| 3 | N/A | • Caplacizumab should NOT be used, regardless of the probability of TTP | Conditional | Low certainty |
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; N/A, not applicable; TPE, therapeutic plasma exchange; TTP, thrombotic thrombocytopenic purpura. Depending on the clinician’s judgment and assessment of the individual patient.
- Zheng XL, et al. J Thromb Haemost. 17 July 2020; doi:10.1111/JTH.15006.
In Settings With Timely Access to ADAMTS13 Activity Testing
Patients with high clinical suspicion of TTP1
- Determined by clinical assessment OR a formal clinical risk assessment method (such as PLASMIC2 or French score3)
- Availability of ADAMTS13 activity results is ideally in <72 hrs; acceptable <7 days1
| Recommendation number | Key recommendations | Strength of recommendation | Evidence grade |
|---|---|---|---|
| 1 | • Acquire a plasma sample and test for ADAMTS13 activity and inhibitor/anti-ADAMTS13 IgG levels before TPE or blood product use • Start TPE and corticosteroids before receiving ADAMTS13 test result • Consider early administration of caplacizumab before receiving ADAMTS13 test results (see Recommendation 5 in Management Guidelines4) • If ADAMTS13 activity <10 IU/dL or <10% of normal (positive result): continue caplacizumab and consider starting rituximabb,c as soon as possible to reduce autoantibody production • If ADAMTS13 activity 10–20 IU/dL or 10–20% of normal (equivocal result): continue or stop TPE, corticosteroids, rituximab, and caplacizumab, based on clinical judgment | Conditional | Low certainty |
- Evidence for specific scores was not evaluated; therefore, no specific scoring system is recommended
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; TPE, therapeutic plasma exchange; TTP, thrombotic thrombocytopenic purpura.
aThe recommendation applies irrespective of the timing and availability of ADAMTS13 results. bThe majority of these patients have antibodies against ADAMTS13. cRituximab is not approved for the treatment of TTP episodes.
- Zheng XL, et al. J Thromb Haemost. 17 July 2020; doi:10.1111/JTH.15006.
- Bendapudi PK, et al. Lancet Haematol. 2017;4(4):e157-64.
- Coppo P, et al. PLoS One. 2010;5(4):e10208.
- Zheng XL, et al. J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010.
Suggested Diagnostic Strategy: High Pretest Probability of aTTP
Patients with high clinical suspicion of aTTP (pretest probability ≥90%), based on clinical assessment OR a formal clinical risk assessment method
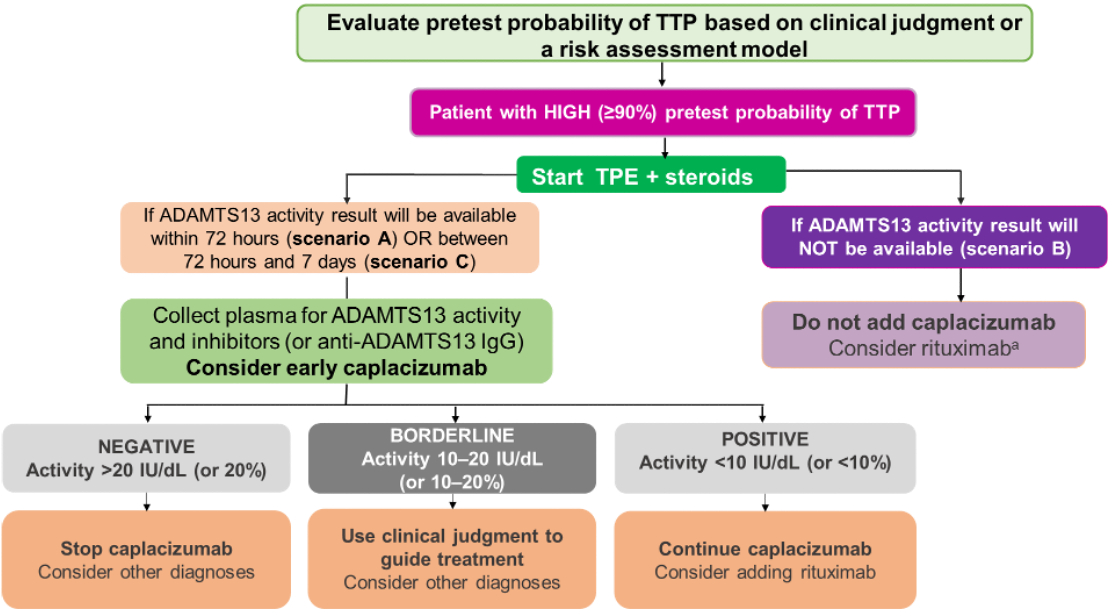
- Determine pretest probability of TTP using clinical judgment or a risk assessment model (PLASMIC or French score)
- If patient has high pretest probability, begin TPE and corticosteroids
- If timely ADAMTS13 testing is available (ideally in <72 hours; acceptable <7 days), acquire plasma samples (before initiating TPE or use of any blood product) to test for ADAMTS13 activity and inhibitor levels
- Consider starting early caplacizumab before ADAMTS13 test results are available
- Upon receiving ADAMTS13 test results: if positive, continue caplacizumab and consider adding rituximab; if borderline, use clinical judgment to guide treatment and consider other diagnoses; if negative, stop caplacizumab and consider other diagnoses
- If ADAMTS13 testing is not available, do not use caplacizumab; consider rituximab
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aTTP, acquired thrombotic thrombocytopenic purpura; IgG, immunoglobulin G; TPE, therapeutic plasma exchange; TTP, thrombotic thrombocytopenic purpura.
aRituximab is not approved for the treatment of TTP episodes.
- Zheng XL, et al. J Thromb Haemost. 17 July 2020; doi:10.1111/JTH.15006.
In Settings With Timely Access to ADAMTS13 Activity Testing
Patients with low or intermediate clinical suspicion of TTP
- Pretest probability determined by clinical assessment OR a formal clinical risk assessment method
| Recommendation number | Key recommendations | Strength of recommendation | Evidence grade |
|---|---|---|---|
| 2 | • Acquire a plasma sample and test for ADAMTS13 activity and inhibitor/anti-ADAMTS13 IgG levels before TPE or blood product use • Consider starting TPE and corticosteroids before receiving ADAMTS13 test results • Do NOT start caplacizumab until ADAMTS13 test results are received • If ADAMTS13 activity <10 IU/dL or <10% of normal (positive result with inhibitors): consider starting caplacizumab and rituximabc (see Recommendation 2 and Recommendation 5 in Management Guidelines2) • If ADAMTS13 activity >20 IU/dL or >20% of normal (negative result): do NOT start caplacizumab and consider other diagnoses • If ADAMTS13 activity 10–20 IU/dL or 10–20% of normal (equivocal result): continue or stop TPE and corticosteroids, or add caplacizumab or rituximab based on clinical judgment | Conditional | Low certainty |
- Evidence for specific scores was not evaluated; therefore, no specific scoring system is recommended
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; TPE, therapeutic plasma exchange; TTP, thrombotic thrombocytopenic purpura.
aThe recommendation applies irrespective of the timing and availability of ADAMTS13 results. bDepending on the clinician's judgment and assessment of the individual patient. cRituximab is not approved for the treatment of TTP episodes.
- Zheng XL, et al. J Thromb Haemost. 17 July 2020; doi:10.1111/JTH.15006.
- Zheng XL, et al. J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010.
Suggested Diagnostic Strategy: Low or Intermediate Pretest Probability of aTTP
Patients with low or intermediate cal suspicion of aTTP (pretest probability <90%), based on clinical assessment OR a formal clinical risk assessment method
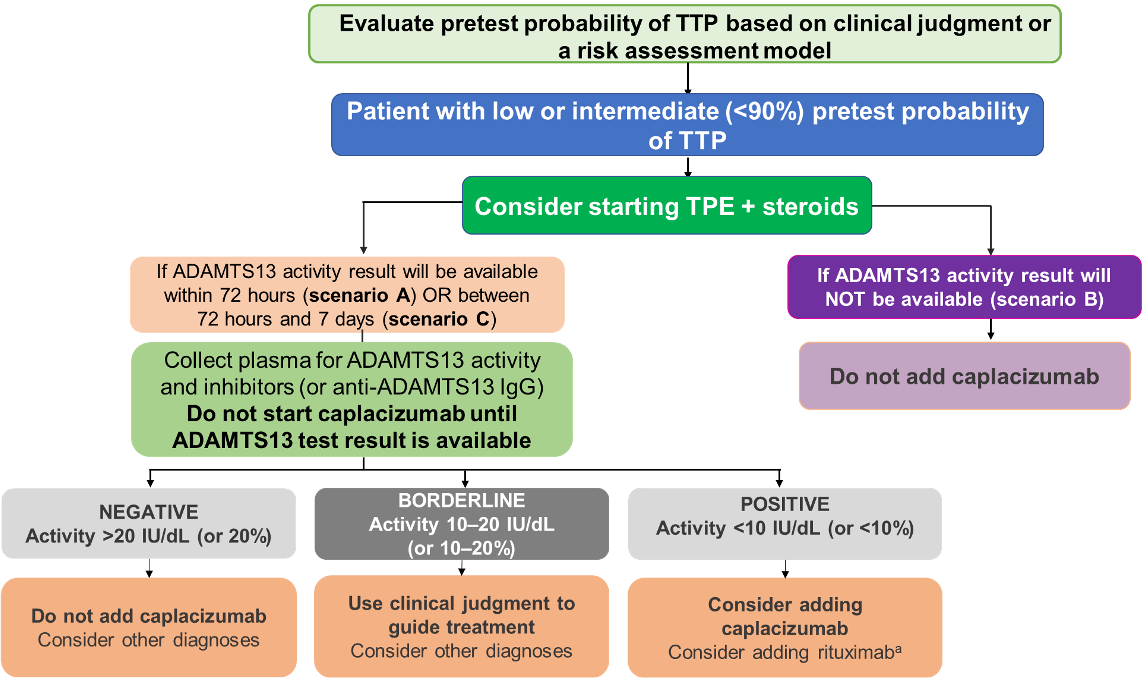
- Determine pretest probability of TTP using clinical judgment or a risk assessment model (PLASMIC or French score)
- If patient has low or intermediate pretest probability (<90%) of TTP, consider early TPE and corticosteroids
- If timely ADAMTS13 testing is available (ideally in <72 hours; acceptable <7 days), acquire plasma samples (before initiating TPE or use of any blood product) to test for ADAMTS13 activity and inhibitor levels
- If timely ADAMTS13 testing is available (ideally in <72 hours; acceptable <7 days), acquire plasma samples (before initiating TPE or use of any blood product) to test for ADAMTS13 activity and inhibitor levels
- If timely ADAMTS13 testing is available (ideally in <72 hours; acceptable <7 days), acquire plasma samples (before initiating TPE or use of any blood product) to test for ADAMTS13 activity and inhibitor levels
- If ADAMTS13 testing is not available, do not use caplacizuma
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aTTP, acquired thrombotic thrombocytopenic purpura; Ig, immunoglobulin G; TPE, therapeutic plasma exchange; TTP, thrombotic thrombocytopenic purpura.
aRituximab is not approved for the treatment of TTP episodes.
- Zheng XL, et al. J Thromb Haemost. 17 July 2020; doi:10.1111/JTH.15006.
In Settings Without Reasonable Access to ADAMTS13 Activity Testing
Regardless of pretest probability of aTTP
| Recommendation number | Key recommendations | Strength of recommendation | Evidence grade |
|---|---|---|---|
| 3 | • Do NOT use caplacizumab | Conditional | Low certainty |
- *The bleeding risk, cost, and resource use of caplacizumab do not justify its use in this setting
- It is important to provide clinicians with timely access to plasma ADAMTS13 activity and antibody testing
- For treatment of relapses in patients with a previous aTTP diagnosis, there is no need for a confirmatory ADAMTS13 test
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aTTP, acquired thrombotic thrombocytopenic purpura.
- Zheng XL, et al. J Thromb Haemost. 17 July 2020; doi:10.1111/JTH.15006.
Summary of aTTP Treatment Recommendations
| Treatment recommendationa | Event | Evidence | Strength of recommendation | Evidence grade |
|---|---|---|---|---|
| 1. Add corticosteroids to TPE | First acute | Few studies with heterogenous populations and varied interventions | Strong | Very low certainty |
| 2. Add rituximabb to corticosteroids and TPE | First acute | Nonrandomized studies with possible selection bias | Conditional | Very low certainty |
| 3. Add corticosteroids to TPE | Relapse | Indirect data from use in first acute events of aTTP | Strong | Very low certainty |
| 4. Add rituximabb to corticosteroids and TPE | First acute | Indirect data from use in first | Conditional | Very low certainty |
| 5. Use caplacizumab | First acute or relapse | Two randomized controlled studies | Conditional | Moderate certainty |
| 6. Use rituximab for prophylaxis | Remission, with low plasma ADAMTS13 activityb | A small nonrandomized study | Conditional | Very low certainty |
| 9. Prophylactic treatment | Pregnancy, with decreased plasma ADAMTS13 activityc | Paucity of data; high value placed on potentially life-saving benefit of prophylaxis | Strong | Moderate certainty |
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aTTP, acquired thrombotic thrombocytopenic purpura; TPE, therapeutic plasma exchange. aRecommendations 7, 8, 10a, and 10b are for treatment of hereditary (congenital) TTP, which is not covered in this presentation.
bRituximab is not approved for the treatment of TTP episodes. cWith no other clinical signs/symptoms.
- Zheng XL, et al. J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010.
First Acute Event of aTTP
| Treatment recommendation | Strength of recommendation | Evidence grade | Considerations | |
|---|---|---|---|---|
| 1. Add corticosteroids to TPE over TPE alone | Strong | Very low certainty | • Moderate reduction in mortality risk with adverse events not severe in the short-term • Supported by small studies with heterogenous populations and varied interventions • Small incremental cost and resource use relative to the potential benefits • No detailed recommendation on dose and types of corticosteroids • No detailed recommendation on dose and types of corticosteroids | |
| 2. Add rituximaba to corticosteroids and TPE over corticosteroids and TPE alone | Conditional | Very low certainty | • Supported by nonrandomized studies that assessed a narrow range of outcomes • Possible concern for selection bias (patients receiving rituximab may have had more severe disease) • Primary effect appears to be on preventing relapse; however, not many patients with aTTP may experience a relapse • Primary effect appears to be on preventing relapse; however, not many patients with aTTP may experience a relapse • Rituximab may be considered for patients with known comorbid autoimmune disorder, although evidence is limited | |
aTTP, acquired thrombotic thrombocytopenic purpura; TPE, therapeutic plasma exchange; TTP, thrombotic thrombocytopenic purpura.
aRituximab is not approved for the treatment of TTP episodes.
- Zheng XL, et al. J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010.
aTTP Relapse
| Treatment recommendation | Strength of recommendation | Evidence grade | Considerations | |
|---|---|---|---|---|
| 3. Add corticosteroids to TPE over TPE alone | Strong | Very low certainty | • May reduce mortality in a life-threatening situation No prohibitive short-term adverse events • Largely informed by indirect data in the setting of the first acute aTTP event • Only single arm and registry data exclusively for treatment of relapses • Serious morbidities associated with repeated use may limit acceptability • Small incremental cost and resource use relative to the potential benefits • Dose and types of corticosteroids cannot be recommended • Corticosteroids should be used with caution in patients susceptible to adverse events | |
| 4. Add rituximaba to corticosteroids and TPE over corticosteroids and TPE alone | Conditional | Very low certainty | • Sparse evidence • Indirect data support the benefit of adding rituximab for preventing relapse • Patients may consider rituximab more acceptable than corticosteroids after the first acute event • Risk of subsequent relapse may be higher in patients who have already relapsed • Primary effect appears to be on preventing relapse; however, not many patients with aTTP may experience a relapse • Rituximab should be considered for patients with a known comorbid autoimmune disorder, although subgroup evidence is limited | |
aTTP, acquired thrombotic thrombocytopenic purpura; TPE, therapeutic plasma exchange; TTP, thrombotic thrombocytopenic purpura.
aRituximab is not approved for the treatment of TTP episodes.
- Zheng XL, et al. J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010.
First Acute Event of aTTP or Relapse
| Treatment recommendation | Strength of recommendation | Evidence grade | Considerations | |
|---|---|---|---|---|
| 5. Use caplacizumab | Only recommendation in the guideline with moderate certainty evidence | • Evidence from two published RCTs, one of which was double-blinded2,3 • Low mortality rate in both treatment groups may not reflect true mortality rates in other patient populations; this suggests possible selection bias or that patients had less severe disease • Data suggest clinically important bleeding side effects with caplacizumab and relapse after stopping drug treatment • Data do not differentiate effects on first and relapse events • Data do not differentiate effects on first and relapse events • Use is conditional on rapid identification of patients with a high likelihood of TTP (ie, evidence of severe plasma ADAMTS13 deficiency and inhibitors present) • Use will depend on local cost considerations and availability | ||
| Conditional | Moderate | |||
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aTTP, acquired thrombotic thrombocytopenic purpura; RCT, randomized controlled trial; TTP, thrombotic thrombocytopenic purpura.
- Zheng XL, et al. J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010.
- Scully M, et al. N Engl J Med. 2019;380(4):335-346.
- Scully M, et al. N Engl J Med. 2019;380(4):335-346.
First Acute Event of aTTP or Relapse
Practical considerations for caplacizumab use1
- Physicians should consider administering caplacizumab treatment before ADAMTS13 activity test results are available (see Recommendations 1–3 in the Diagnosis Guidelines2)
- Caplacizumab should be used only under the guidance of a trained TTP expert (hematologist or transfusion medicine specialist); however, few clinicians are familiar with caplacizumab use and monitoring1
- Clinicians must develop better understanding of the mode of action for caplacizumab1
- Caplacizumab does not correct underlying ADAMTS13 deficiency; therefore, other treatments might be required1
- Discontinuing caplacizumab after platelet count normalization with persistently low ADAMTS13 activity <10 IU/dL may result in an exacerbation1,3
- Implementation of caplacizumab therapy is dependent on clinicians’ access to timely and reliable ADAMTS13 testing1
- Further data on the optimal use of caplacizumab and cost-effective strategies are needed
- Caplacizumab use in these patient populations is considered feasible for patients in an outpatient or in-home setting via subcutaneous administration1
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aTTP, acquired thrombotic thrombocytopenic purpura; RCT, randomized controlled trial; TTP, thrombotic thrombocytopenic purpura.
- Zheng XL, et al. J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010. 2. Zheng XL, et al. J Thromb Haemost. 17 July 2020; doi:10.1111/JTH.15006. 3. Scully M, et al. N Engl J Med. 2019;380(4):335-346.
Patients With aTTP in Remission
- Patients with aTTP who are in remission and still have low plasma ADAMTS13 activity but no other clinical signs/symptoms
| Treatment recommendation | Strength of recommendation | Evidence grade | Considerations | |
|---|---|---|---|---|
| 6. Use rituximaba over not using rituximab for prophylaxis | Conditional | Very low certainty | • A small nonrandomized study suggests that rituximab reduces the rate of relapses • No clear differentiation between first and subsequent remissions • Rituximab does not appear to affect survival • Issues surround cost, resources, and continued monitoring • Rituximab prophylaxis without ADAMTS13 testing is not an evidence-based strategy | |
Practical considerations for rituximab use
- There is no guidance on appropriate interval for ADAMTS13 testing for patients in remission
- This strategy may not be acceptable or feasible in all centers or patients
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aTTP, acquired thrombotic thrombocytopenic purpura; TTP, thrombotic thrombocytopenic purpura.
aRituximab is not approved for the treatment of TTP episodes.
- Zheng XL, et al. J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010.
Patients With aTTP Who Are Pregnant
- Patients with aTTP who are in remission and still have low plasma ADAMTS13 activity but no other clinical signs/symptoms
| Treatment recommendation | Strength of recommendation | Evidence grade | Considerations | |
|---|---|---|---|---|
| 9. Prophylactic treatment | Strong | Very low certainty | • Potentially life-saving benefits of prophylactic treatment likely outweigh the risks • There is a paucity of data on the effect of available treatment regimens • May reduce maternal and infant mortality | |
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aTTP, acquired thrombotic thrombocytopenic purpura.
- Zheng XL, et al. J Thromb Haemost. 15 July 2020; doi:10.1111/JTH.15010.
PLASMIC and French Scores For Predicting ADAMTS13 Deficiency
| Parameters | French score | PLASMIC score |
|---|---|---|
| Platelet count | <30 × 109/L (+1) | <30 × 109/L (+1) |
| Serum creatinine level | <2.26 mg/dL (+1) | <2.0 mg/dL (+1) |
| Hemolysis | * | +1 |
| No active cancer in previous year | * | +1 |
| No history of solid organ or SCT | * | +1 |
| INR <1.5 | * | +1 |
| MCV <90 fL | NA | +1 |
| Likelihood of severe deficiency of ADAMTS13 activity (<10%) | 0: 2% | 0–4: 0–4% |
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aTTP, acquired thrombotic thrombocytopenic purpura; INR, international normalized ratio; MCV, mean corpuscular volume; NA, not available; SCT, stem cell transplantation; TMA, thrombotic microangiopathy.
French score considered patients with TMA that included hemolysis and schistocytes in their definition and assumed that there was no history or clinical evidence for associated cancer, transplantation or disseminated coagulation. Therefore, these items were intrinsic to the scoring system. NA and MCV were not incorporated in the French score. Table adapted from Joly BS, et al.
- Zheng XL, et al. J Thromb Haemost. 15July 2020; doi:10.1111/JTH.15006.
- Joly BS, et al. Expert Rev Hematol. 2019;12(6)383-395.
To report an adverse event or drug reaction please contact us on
Email: Gulf.Pharmacovigilance@sanofi.com | www.sanofi.com
For further medical information please contact: medical-information.gulf@sanofi.com | In UAE:
Toll free number 800 MEDICAL
.png/jcr:content/jcr_content%20(26).png)