Odyssey outcomes

Odyssey Outcomes: Addition of PCSK9i to background Statin therapy further reduces MACE.
Study Design¹
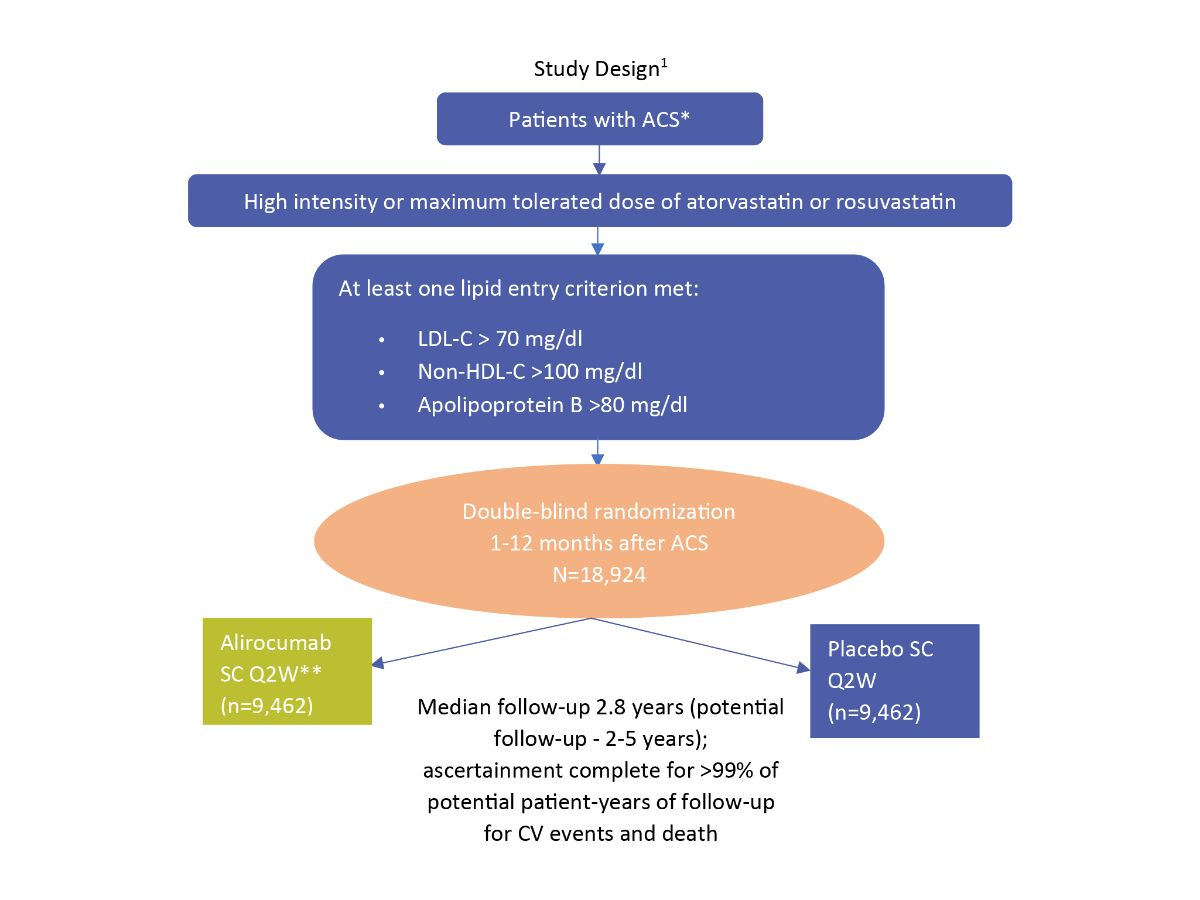
*1-12 months from index ACS event
**Blinded adjustment of alirocumab dose to target achieved LDL-C 25- 50 mg/dl and avoid sustained levels <15 mg/dl
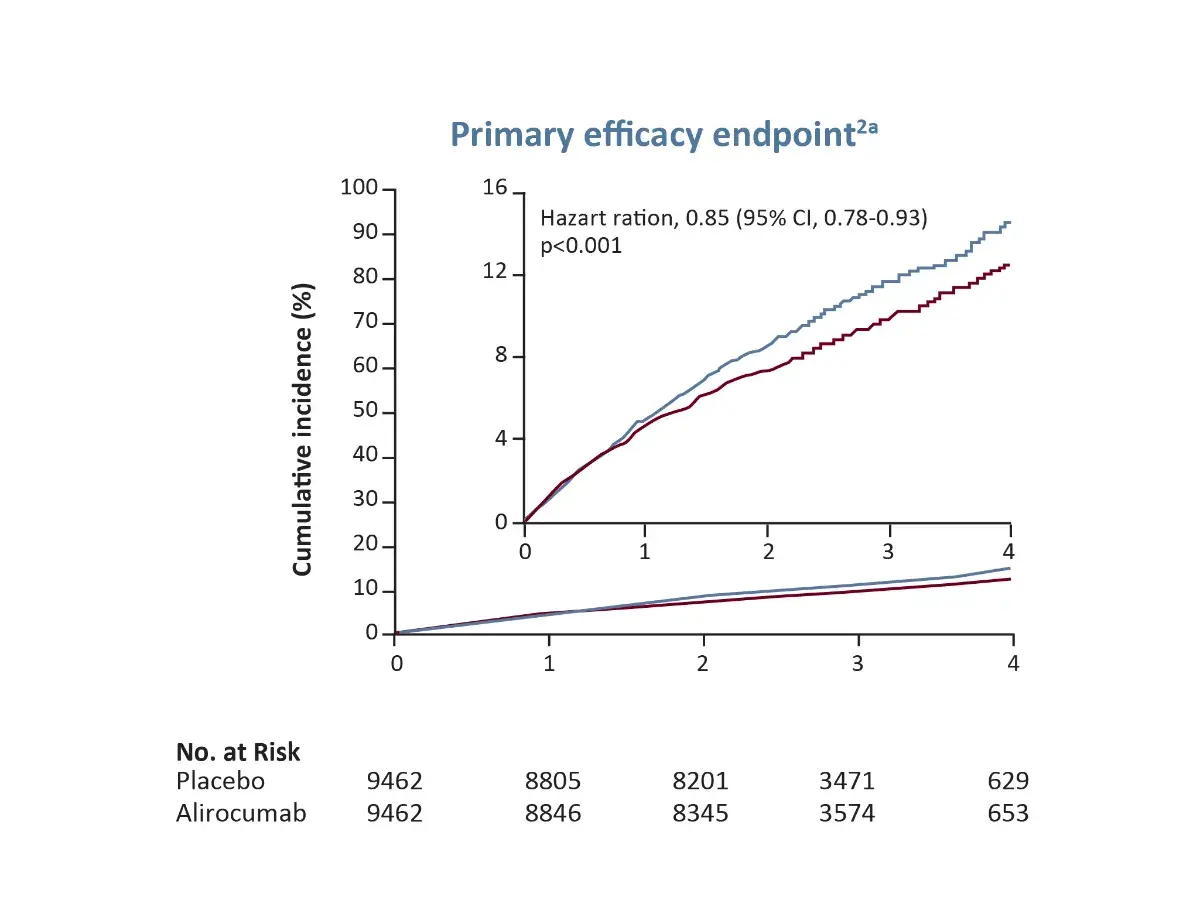
aPrimary efficacy endpoint (a composite of death from CHD, nonfatal MI, fatal or nonfatal ischaemic stroke, or unstable angina requiring hospitalization)
HDL-C, High-density lipoprotein cholesterol; Q2W, every 2 weeks; SC, subcutaneous
Major Primary endpoints¹
The primary endpoint was a composite of:
- Death from coronary heart disease
- Nonfatal myocardial infarction
- Fatal or nonfatal ischemic stroke
- Unstable angina requiring hospitalization.
Major Secondary efficacy Endpoints²
Tested in the following hierarchical sequence:
- CHD event: CHD death, nonfatal MI, unstable angina requiring hospitalization, or ischaemia-driven coronary revascularization*
- Major CHD event: CHD death or nonfatal MI
- CV event: CV death, nonfatal CHD event, or nonfatal ischaemic stroke
- All-cause death, nonfatal MI, nonfatal ischaemic stroke
- CHD death
- CV death
- All-cause death
*Revascularization performed because of recurrent ACS, new or progressive symptoms of myocardial ischemia or new or progressive abnormalities on functional testing, except revascularization due to restenosis at a prior coronary intervention site.
Odyssey Outcomes: Addition of PCSK9i to background Statin therapy further reduces MACE
Safety: Incidence or adverse events and laboratory abnormalities was similar in the alirocumab group and the placebo group, apart from local injection-site reaction (3.8% in alirocumab group vs 2.1% in the placebo group, p<0.001).
ᵃ Primary efficacy endpoint (a composite of death from CHD, nonfatal MI, fatal or nonfatal ischaemic stroke, or unstable angina requiring hospitalization)
CHD, Coronary Heart Disease; CI, Confidence Interval; MI, Myocardial Infarction
Primary MACE, All-cause death and safety
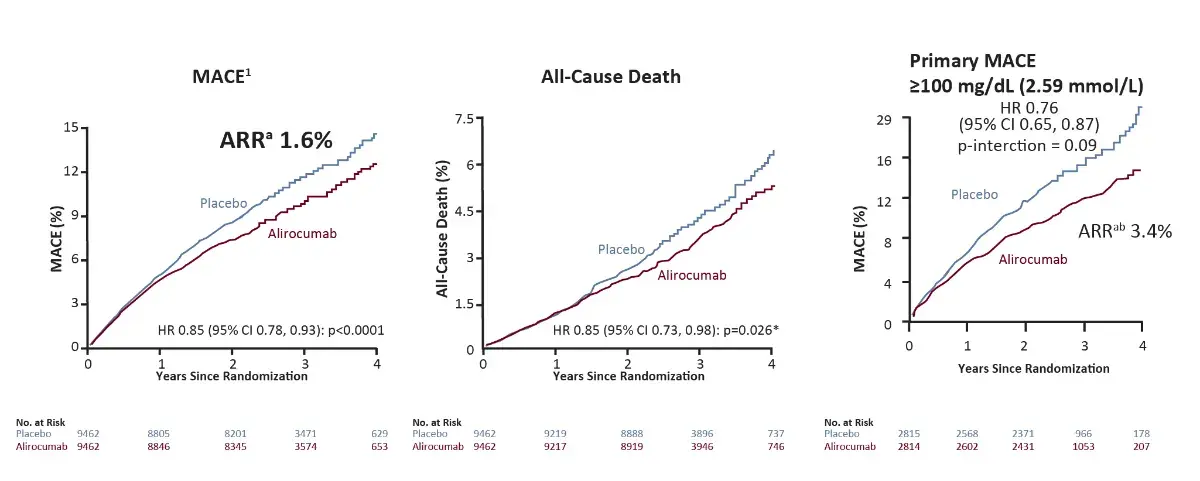
Safety
Similar incidence of adverse events and laboratory abnormalities in the alirocumab group and the placebo group, except for local injection-site reactions (3 .8% in the alirocumab group vs 2.1% in the placebo group, p<0.001)¹
*Nominal p-value; ᵃBased on cumulative incidence; bp-interaction value for ARR <0.001 in post-hoc analysis
ARR, Absolute Risk Ratio; HR, Hazard ratio
All-cause death⁴
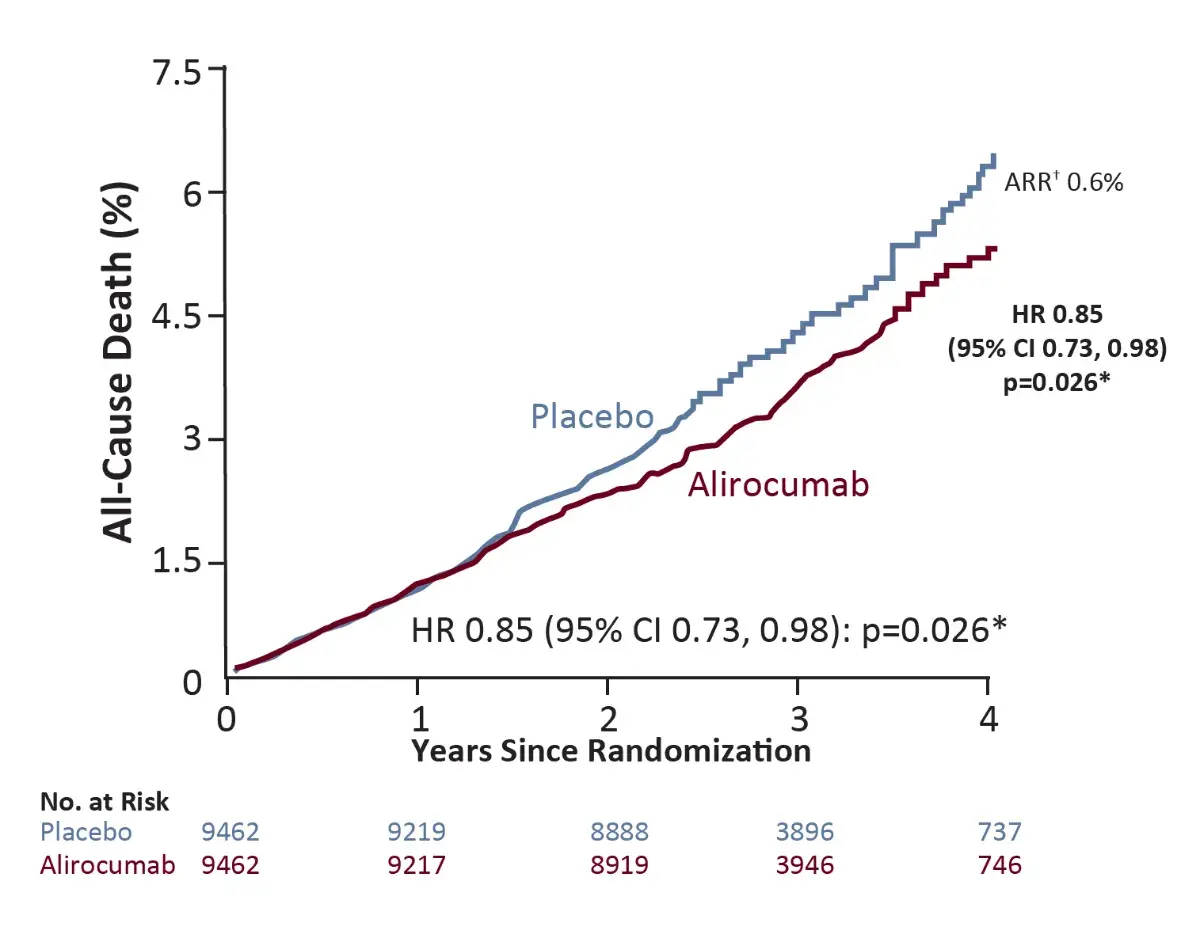
*Nominal p-value
†Based on cumulative incidence
Primary Analysis Conclusions¹
- The primary results show that, compared with placebo in patients with recent ACS, alirocumab 75 or 150 mg SC twice a week targeting LDL-C levels 25-50 mg/dl reduced MACE, MI*, ischemic stroke* and was associated with a lower rate of all-cause death
- Among patients with ACS and baseline LDL-C >100 mg/dl, alirocumab was well tolerated, reduced MACE by 24% (absolute risk reduction [ARR] 3.4%), and was associated with a lower rate of all-cause death compared with placebo
*Nominally significant as per hierarchical testing.
- Shwartz GG, et al. NEJM. 2018; 397:2097-107
- Shwartz GG, et al. Am Heart J. 2014; 168:682-689.e1
- Steg PG, et al. Circulation. 2019; 140(2):103-12
- Shwartz GG, et al. NEJM. 2018; 397:2097-107.Supplementary Appendix