iGlarLixi Enables Safe Ramadan Fasting for Most T2D Patients
Introduction
- Most Muslim adults with type 2 diabetes (T2D) choose to fast during Ramadan, with >85% fasting for ≥15 days days1-3
- The incidence of hypoglycaemia increases during Ramadan compared with prepre-Ramadan, and adjustments to diabetes treatment must be considered to prevent severe hypoglycaemia3
- Separate administration of basal insulin + the glucagon glucagon-like peptide peptide-1 receptor agonist, lixisenatide, has been shown to demonstrate lower risk of hypoglycaemia during Ramadan versus sulfonylureas + lixisenatide lixisenatide4
- In the SoliRam study, the safety and effectiveness of iGlarLixi (a fixed fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide) were evaluated in adults with T2D during Ramadan
Objective
To assess the safety and effectiveness of iGlarLixi in people with T2D fasting during Ramadan
Study design
SoliRam was a multicentre, multinational, prospective, single-arm, real-world observational study during Ramadan 2020 (Wave 1) and 2021 (Wave 2) (Figure 1)
Figure 1: SoliRam study design and endpoints

Population
Adults with T2D treated with iGlarLixi for ≥3 months prior to study start who intended to fast for ≥15 days during Ramadan*
Primary endpoint
- Proportion of participants experiencing at least one documented (<70 mg/dL [<3.9 mmol/L]) and/or severe symptomatic hypoglycaemia event during pre pre-Ramadan, Ramadan, post post-Ramadan and the whole study period period‡
Secondary endpoints
- Changes in HbA HbA1c, FPG and body weight from pre-to post post-Ramadan
- Changes in iGlarLixi and other non-insulin antihyperglycaemic treatment
- AEs and serious AEs
*Participants who entered Wave 1 were not included in Wave 2; †Participant follow follow-up was preferably 1 month after the end of Ramadan; ‡Whole study period: pre-Ramadan to post-Ramadan.
During the study, iGlarLixi and concomitant treatments were adjusted by physicians as per routine practice AE, adverse event; DAR, Diabetes and Ramadan; FPG, fasting plasma glucose; IDF, International Diabetes Federation; iGlarLixi, a fixed fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide, T2D, type 2 diabetes
- Participants were enrolled from Egypt, Indonesia, Israel, Kuwait, Lebanon, Malaysia, Philippines, Saudi Arabia and the United Arab Emirates
- This analysis presents descriptive statistics from the entire SoliRam study
Results
Participant baseline characteristics
- Overall, 420 people with T2D were eligible. Baseline characteristics are presented in Table 1
- In total, 409 participants were assessed during the Ramadan period
Table 1: Participant baseline characteristics
| Baseline characteristics | N=420* |
| Age (years) ≥65 years | 57.1 ± 9.8 101 (24.0) |
| Female | 188 (44.8) |
| BMI (kg/m2) Body weight (kg) | 30.9 ± 5.3 86.5 ± 14.9 |
| Diabetes duration (years) ≥10 years | 12.1 ± 6.4 246 (58.6) |
| HbA1c(%) pre-Ramadan period | 8.2 ± 1.2 |
| FPG (mg/dL) pre-Ramadan period | 140 ± 38 |
| Duration of iGlarLixi treatment (months months†) iGlarLixi started in insulin-naïve ve participants iGlarLixi started after previous insulin treatment | 6.2 ± 4.2 174 (41.9) 241 (58.1) |
| Concomitant non-insulin antihyperglycaemic treatment pre-Ramadan period period ‡ Biguanides Sulfonylureas SGLT-2 inhibitors Thiazolidinedione Glinides DPP-4 inhibitors | 359 (85.5) 234 (55.7) 188 (44.8) 173 (41.2) 18 (4.3) 7 (1.7) 1 (0.2) |
*Eligible population; †Up to signing of informed consent form; ‡A participant can be included in several categories Data are mean ± SD or n (%)
BMI, body mass index; DPP-4, dipeptidyl peptidase-4; FPG, fasting plasma glucose; SD, standard deviation; SGLT-2, sodium-glucose co-transporter-2
Antihyperglycaemic therapies during Ramadan
- At the selection visit (n=420), iGlarLixi injection time was at breakfast, lunch or dinner for 41.2%, 36.2% and 22.6% of participants, respectively
- During Ramadan most participants (88.8%) took iGlarLixi at Iftar
- iGlarLixi mean ± standard deviation (SD) daily dose was 25.8 ± 11.0 U pre-Ramadan and 24.8 ± 9.9 U during Ramadan
- Minimal adjustments were made to antihyperglycaemic therapies from pre-Ramadan to during Ramadan. Oral antihyperglycaemic drugs were used by 83.8% of participants during Ramadan (54.5% biguanides, 43.8% sulfonylureas and 40.5% sodium sodium-glucose co-transporter-2 [SGLT SGLT-2] inhibitors)
Fasting during Ramadan
- Most participants (96.9%) were able to fast for ≥25 days (mean ± SD, 28.8 ± 2.7 days), with 92.4% not breaking their fast during the whole Ramadan period
- Of the 31 (7.6%) participants who did break their fast, four (12.9%) reported hypoglycaemia as the reason
Hypoglycaemia during Ramadan
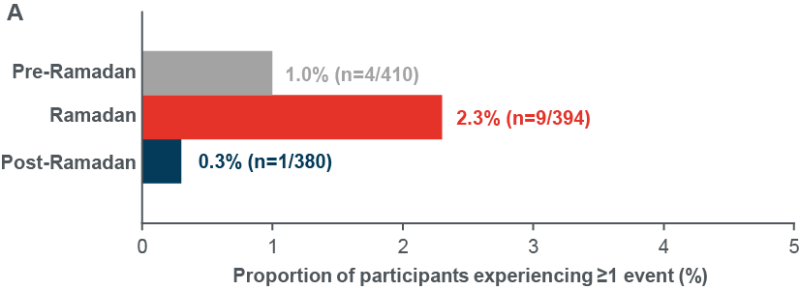
- Primary endpoint: The number of participants reporting ≥1 severe and/or documented symptomatic hypoglycaemia events <70 mg/dL (<3.9 mmol/L) was low throughout the whole study ( Figure 2A)
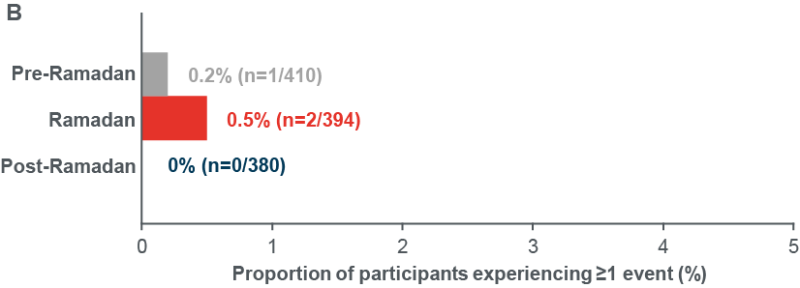
- The number of participants reporting ≥1 severe and/or documented symptomatic hypoglycaemia events <54 mg/dL (<3.0 mmol/L) was also low throughout the study ( Figure 2B)
- No severe hypoglycaemia events occurred during the whole study period
Figure 2: Incidence of documented symptomatic and/or severe hypoglycaemia (A) <70 mg/dL (<3.9 mmol/L), (B) <54 mg/dL (<3.0 mmol/L)
Secondary endpoints
- HbA1c , FPG and body weight all improved from pre-to post-Ramadan (Figure3)
- The proportion of participants reaching HbA1c <7 % increased from 7.9% pre-Ramadan to 28.6% post-Ramadan (evaluable population, n=343)
Figure 3: Changes in (A) HbA1c, (B) FPG and (C) body weight between pre-and post-Ramadan periods
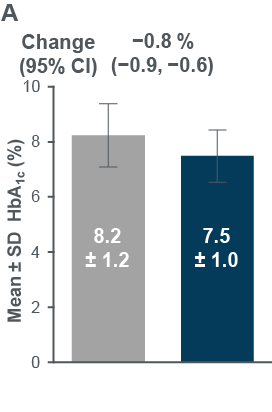
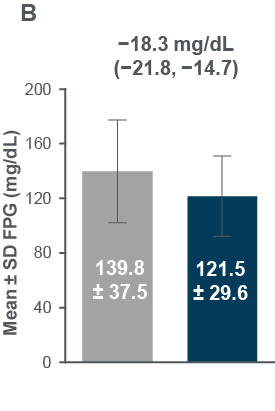
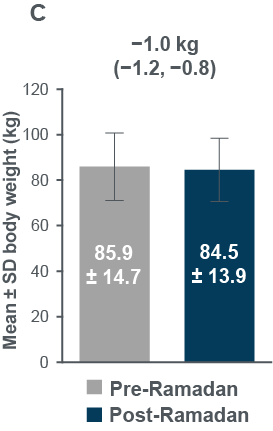
Evaluable population: HbA1c, N=343; FPG, N=334; Eligible population: body weight, N=356 (post-Ramadan observed data)
CI, confidence interval; FPG, fasting plasma glucose, SD, standard deviation
Adverse events
- Overall, 4.7% of participants (n=20) reported adverse events (AEs) of any cause ( Table 2)
Table 2: Adverse events
| Adverse events | N=428* |
| Any | 20 (4.7) |
| Any serious AE | 0 (0.0) |
| Any treatment treatment-related AE | 0 (0.0) |
| Any AE leading to permanent treatment discontinuation | 1 (0.2) |
| Any AE leading to death | 0 (0.0) |
*Included population. Data are n (%) AE, adverse event
- Gastrointestinal AEs were reported by four (0.9%) participants
- The AE leading to discontinuation was asthenia, and treatment was stopped by the participant without physician advice
Incidence of other Hypoglycaemia events
Incidence of documented (<54 mg/dL [<3.0 mmol/L]) symptomatic and/or severe hypoglycaemia events
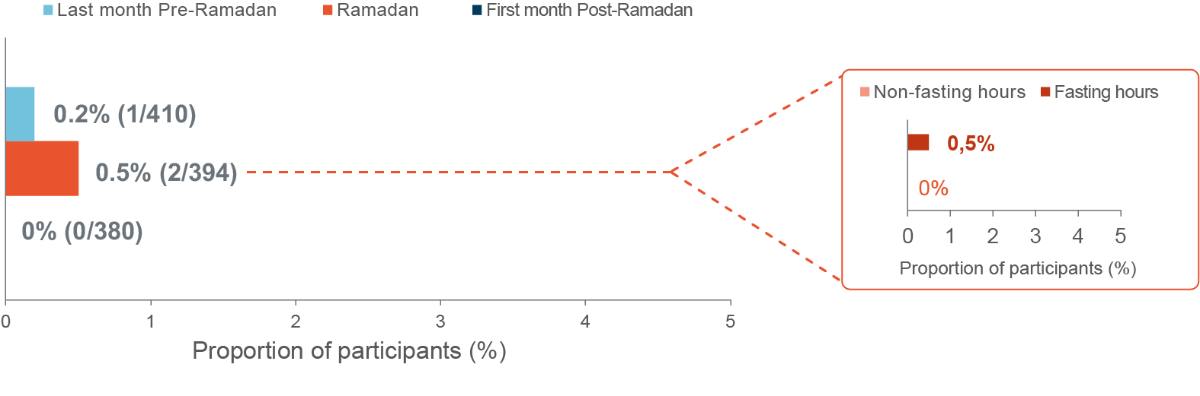
Eligible population. n=410 Last month Pre-Ramadan, n=394 Ramadan and n=380 First month Post-Ramadan Poster presentation EDEC 2022, Mohamed Hassanein et al, EDEC2022 (edec-uae.com) last accessed 27th February 2022.
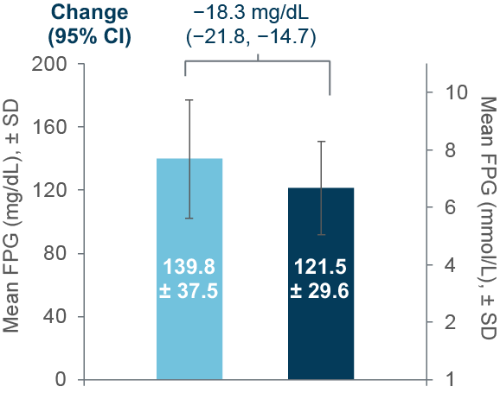
Changes in FPG
.png)
Improvements were also observed in FPG
FPG n=334 for both Pre- and post-Ramadan; Body weight n=406 (Pre-Ramadan), n=356 (Post-Ramadan) CI, confidence interval; FPG, fasting plasma glucose; SD, standard deviation.
Discussion
- In this real real-world study of adults with T2D from diverse regions fasting during Ramadan, over 90% of participants treated with iGlarLixi were able to fast for the entire month of Ramadan, with few participants experiencing severe and/or documented (<70 mg/dL [<3.9 mmol/L]) symptomatic hypoglycaemia
- Adjustments to antihyperglycaemic regimens were minimal and HbA1c levels did not increase over Ramadan
- No participants experienced severe hypoglycaemia
Conclusion
iGlarLixi may be a suitable and effective treatment option for people with T2D who intend to fast during Ramadan
- International Diabetes Federation, Diabetes and Ramadan 2021
- Babineaux SM et al. Diabetic Med 2015;32:819 819–28
- Hassanein M et al. Diab Res Clin Pract 2019;151:275 275–84
- Hassanein M et al. Diab Res Clin Pract 2019;150:331 331–41