EPOS2020/EUFOREA Expert Opinion on Defining Disease States and Therapeutic Goals in CRSwNP
An expert panel convened by EPOS2020 and EUFOREA proposed standardized definitions for disease control, remission, and progression in CRSwNP. This initiative aims to harmonize and optimize the standard of care for patients with CRSwNP.
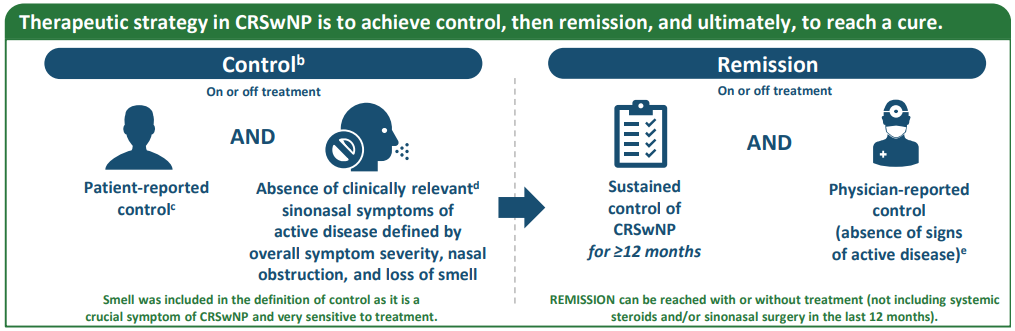
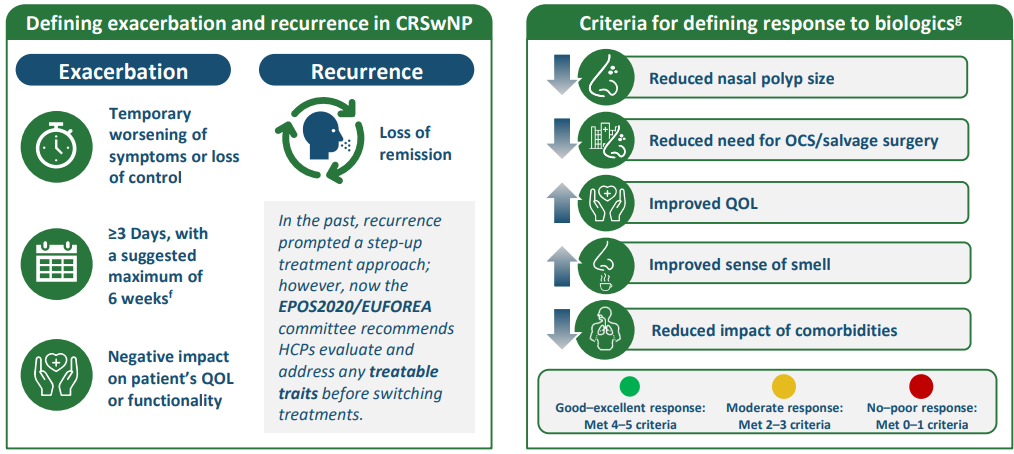

aNo positioning of biologics was discussed. bUncontrolled CRSwNP was defined as “patient-reported lack of control and the presence of clinically relevant sinonasal symptoms of active disease (defined as overall symptom severity, nasal obstruction, and smell).” cBecause of a lack of research evaluating control in patients with CRSwNP alone, no exact cutoff was provided for patient-reported control. Advisors recommended asking patients whether their individual symptoms were controlled or burdensome with a binary (yes/no) answer. dFor research purposes, symptoms were not considered clinically relevant/bothersome when a patient’s VAS score was ≤5 cm for a particular symptom, as a previous study showed that a VAS score of 5 cm for overall symptoms severity best distinguished between patients whose CRS symptoms affected their QOL. ePreferably evaluated by nasal endoscopy. fThere was no unanimous agreement on the length of an exacerbation, but a suggestion of 6 weeks maximum was accepted. gThe 2024 definition of treatment response emphasizes individual therapy optimization (evaluate alternative diagnosis, assess treatable traits, and consider salvage surgery or biologic switch) and provides reference values for criteria compared with the definition provided in 2023.1,2 CRS, chronic rhinosinusitis; CRSwNP, chronic rhinosinusitis with nasal polyps; EPOS, European Position Paper on Rhinosinusitis and Nasal Polyps; EUFOREA, European Forum for Research and Education in Allergy and Airways Diseases; HCP, healthcare professional; OCS, oral corticosteroids; QOL, quality of life; VAS, visual analog scale. 1. Fokkens WJ, et al. Rhinology. 2024. doi:10.4193/Rhin23.415. 2. Fokkens WJ, et al. Rhinology. 2023;61:194–202.
