
SEROGROUP A is responsible for majority of MENINGOCOCCAL MENINGITIS outbreaks in India1

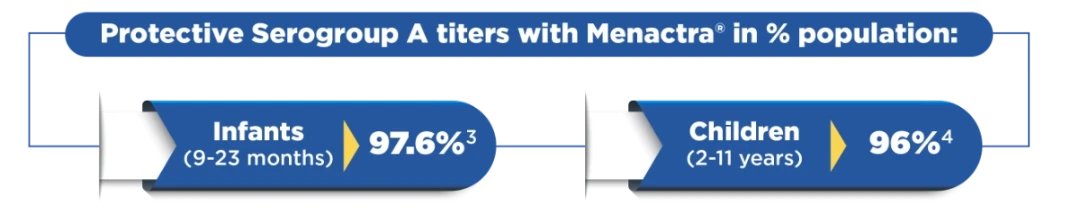
Offers high immuno-protection against serogroup A2
Epidemiological profiles of meningococcal meningitis outbreaks in India:
| Study ID | Confirmed cases | Year | Group | |||
| Ahuja & Singh (1935) | - | 1934 | 30 strains tested, all types I & III† (Group ‘A’) |
|||
| Ghosh (1970) | 23 | 1966-67 | All group ‘A’ | |||
| Prakash and Ghosh | 31 | 1966-67 | All group ‘A’ Ray (1968) | |||
| Bhatia et al. (1968) | 166 | 1966-67 | All group ‘A’ | |||
| Dubey et al. (1986) | 24 | 1985 | 18 group ‘A’, 6 group ‘C’ | |||
| Sarkar et al. (1987) | 174 | 1985 | All group ‘A’ | |||
| Deorari et al. (1987) | 20 | 1985-86 | All group ‘A’ | |||
| Talukdar et al. (1988) | 100 | 1985 | All group ‘A’ | |||
| Singh et al. (1988) | 34 | 1985-86 | All group ‘A’ | |||
| Ayyagari et al. (1987) | 45 | 1985-86 | 37 group ‘A’, 7 ‘C’, 1‘non-A, non-C’ |
|||
| Annapurna et al. (1989) | 27 | 1985-86 | All group ‘A’ | |||
| Bhavsar et al. (1989) | 66‡ | 1985-87 | Confirmed as group ‘A’ | |||
| Sehgal (1987) | 2 | 1987 | Both group ‘A’ | |||
| Mirdha et al. (1991) | 12 | 1988-89 | All group ‘A’ | |||
| Saha et al. (2006) | 1 | 2005 | Group ‘A’ | |||
| Nair (2009) | 32 | 2005-06 | All 26 strains group ‘A’§ | |||
| Duggal et al. (2007) | 124 | 2005-06 | 42 strains confirmed as group ‘A’¶ |
|||
| Kumar et al. (2008) | 33 | 2005-07 | All group ‘A’ | |||
*Confirmed cases: clinical features plus culture +ve or antigen detection +ve for Neisseria meningitidis. †This refers to a historical typing system (Gordon and Murray). ‡This study includes confirmed cases and probable cases (clinical features plus: Gram-negative diplococci on smear or a petechial rash). § Multi-locus Sequence Typing analysis suggests these isolates were similar to the outbreak strains from Bangladesh (2002) and Nigeria (2003). ¶A further 63 samples agglutinated with combined ACYW135 but were not tested for a single serotype..
Menactra® dosing schedule:

Infants & children
(9-23 months): 2 doses
(atleast 3 months apart)

Individuals
(2-55 years): 1 dose
This information is only for healthcare professionals.
- Sinclair D, Preziosi MP, Jacob John T, Greenwood B. The epidemiology of meningococcal disease in India. Trop Med Int Health. 2010 Dec; 15(12):1421-35. doi: 10.1111/j.1365-3156.2010.02660.x. Epub 2010 Nov 5. PMID: 21054695. 2, Halperin S, Gupta A, Jeanfreau R, Klein N, Reisinger K, Walter E, et al. Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2-10 years of age. Vaccine. 2010 Nov 23;28(50):7865-72. Available at: https://pubmed.ncbi.nlm.nih.gov/20943209/3. Bakul Javedekar et al. Safety and Immunogenicity of Two Doses bf a Quadrivalent Meningococcal Polysaccharide Diphtheria Toxoid Conjugate Vaccine in Indian and Russian Children Aged 9 to 17 Months, Received: November 23. 2017: Initial review: March 05, 2018; Accepted: August 27, 2018 4. Yadav, S., Manglani, M.V., Ashwath Narayan, D.H. et al. Indian Pediatr (2014) 51: 451. https://doi.org/10.1007/s13312-014-0435-7 Please visit https://www.sanofi.in/en/science-and-innovation/ for-healthcareprofessionals /product-information for PI/API




