Protection from vaccine preventable disease: A prerequisite for optimal learning1

Download Corner
School age vaccination- Need of hour

Ensuring children in formal education or care settings are protected against all VPDs is important not only for the health of the child, their family and community, but also for a healthy care/school environment, which is a prerequisite for optimal learning.1
A comprehensive school-based approach to deliver immunization services and improve vaccination coverage in school-aged children is consistent with the Global Vaccine Action Plan (GVAP)and Immunization Agenda 2030 (IA2030) strategic priorities to establish platforms for life course vaccination. Studies have shown that measles vaccination, in particular, is associated with improved cognitive functioning and schooling outcomes. Furthermore, childcare and school settings can easily facilitate the transmission of VPDs. Minimizing immunity gaps in these populations and ensuring school-aged children are protected is therefore important for the health of child, their family and community, as well as a healthy school environment, which is a pre-requisite for optimal learning.2
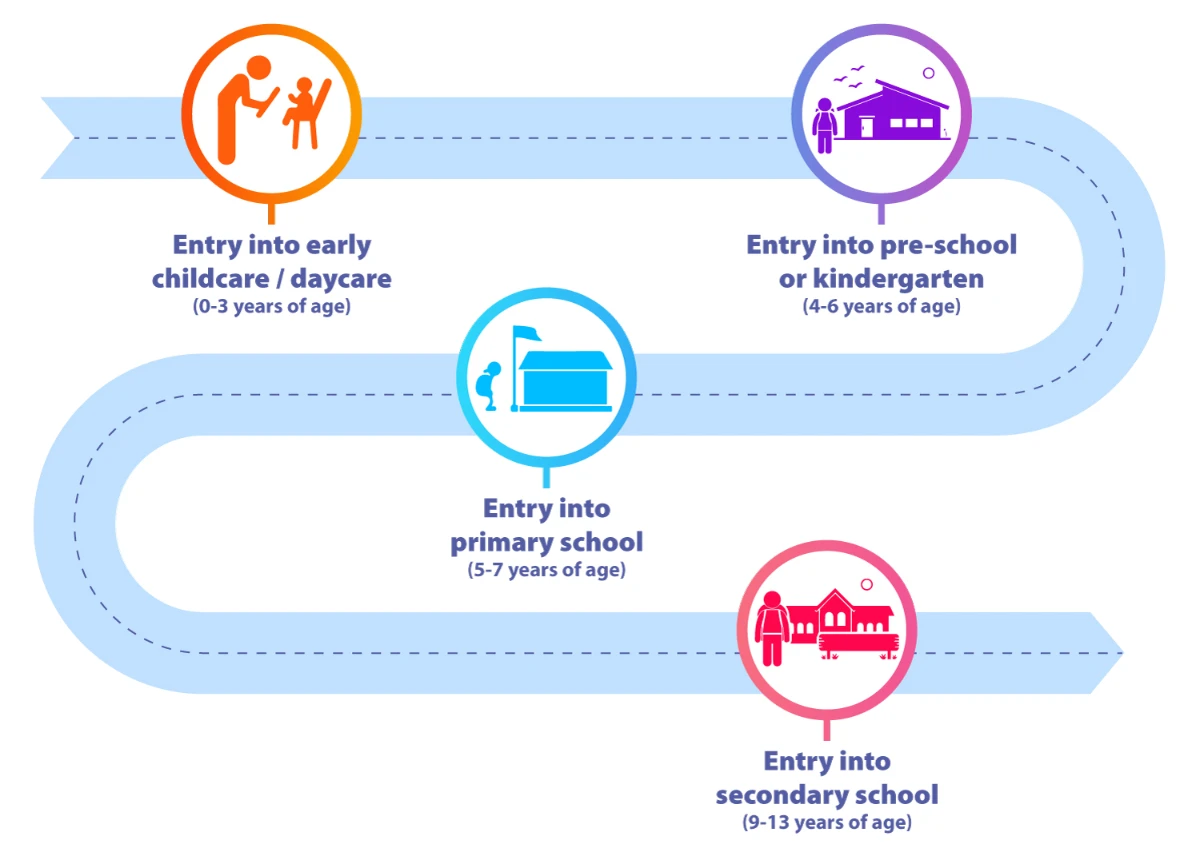
Checking of vaccination status at entry to, or during, childcare, pre-school or primary school is a strategy that has been widely recommended by the World Health Organization (WHO) and others, as a way to improve coverage of routine vaccinations.1 Global strategic plans and guidance for the elimination of measles and rubella and maternal neonatal tetanus call for strengthening of the role of schools in the delivery of routinely recommended vaccines and checking vaccination status at entry to, or during school, to achieve and sustain elimination of these vaccine-preventable diseases (VPDs).2
There are opportunities to check vaccination history at entry to different stages of education...
A child's vaccination journey as per latest ACVIP immunization schedule1

*Vaccines used in special situations
BD- Birth Dose, ACVIP- Advisory Committee on Vaccines & Immunization Practices; @- Typhoid Conjugate vaccine can be administered between 6-9 months. BCG- Bacille Calmette Guerin vaccine; OPV - Oral Polio Vaccine; Hep B – Hepatitis B; DTaP/ DTwP - Diphtheria-Tetanus acellular Pertussis /Diphtheria Tetanus whole cell Pertussis; IPV- Injectable Polio Vaccine; Hib- Haemophilus influenzae type b; Rota- Rotavirus; PCV- Pneumococcal Conjugate Vaccine; PPSV- Pneumococcal Polysaccharide vaccine; IIV- Inactivated Influenza Vaccine; MMR- Measles Mumps Rubella; HepA- Hepatitis A; HPV- Human Papillomavirus; Tdap- Tetanus and diphtheria toxoids with acellular pertussis; MCV: Meningococcal Vaccine; JE: Japanese Encephalitis
(a)To be given within 24 h after birth. When this is missed, it can be administered at first contact with health facility; (b) An extra dose of Hepatitis B vaccine is permitted as part of a combination vaccine when use of this combination vaccine is necessary; (c) IPV can be given as part of a combination vaccine; (d) 3rd dose of Rota vaccine is not necessary for RV1; (e) Influenza vaccine should be started after 6 mo of age, 2 doses 4 wks apart, usually in the pre-monsoon period. At other times of the year, the most recent available strain should be used. Annual influenza vaccination should be continued, for all, till 5 y of age; after the age of 5y, this vaccine is recommended in the high-risk group only; (f) Single dose is to be given for the live attenuated Hepatitis A vaccine. The inactivated vaccine needs two doses; (g) 2nd dose of Varicella vaccine should be given 3-6 mo of age after dose 1. However, it can be administered anytime 3 mo after dose 1 or at 4-6 y; (h) Tdap should not be administered as the second booster of DPT at 4-6 y. For delayed 2nd booster, Tdap can be given after 7 y of age. A dose of Tdap is necessary at 10-12 y, irrespective of previous Tdap administration. If Tdap is unavailable/ unaffordable, it can be substituted with Td; (i) Before 14 completed years, HPV vaccines are recommended as a 2-dose schedule, 6 mo apart; (j) From 15th y onwards and the immunocompromised subjects at all ages, HPV vaccines are recommended as a 3-dose schedule, 0-1-6 (HPV2) or 0-2-6 (HPV4); (k) MenACWY-DT is approved in a 2-dose schedule between 9-23 mo. Minimum interval between two doses should be 3 mo. MenACWY-CRM is also recommended as a single dose schedule after 2 y of age. (l) Due to the nature of rabies (an infectious zoonotic viral disease that is almost always fatal following the onset of clinical symptoms2), there is no defined age indication for vaccine use. This is in alignment with ACVIP’s recommendation for rabies vaccine use across all children aged 0 through 18 years in special situations1
- Indian Academy of Pediatrics (IAP) Advisory Committee on Vaccines and Immunization Practices (ACVIP): Recommended immunization schedule (2020-21) and update on immunization for children aged 0 through 18 years. [cited 2022Nov23]. Available from: https://www.indianpediatrics.net/jan2021/jan-44-53.html 2. World Health Organization. Rabies vaccines: WHO position paper, April 2018 - Recommendations. Vaccine. 2018 Sep 5;36(37):5500-5503.
- World Health Organization; https://www.who.int/teams/immunization-vaccines-and-biologicals/essential-programme-on-immunization/integration/school-vaccination#my_anchor ; Accessed on 8th June 2023
- World Health Organization. Global Consultation On Implementing Vaccination Checks At School; Kuala Lumpur, Malaysia, 26-28 Nov 2019
- Indian Academy of Pediatrics (IAP) Advisory Committee on Vaccines and Immunization Practices (ACVIP): Recommended immunization schedule (2020-21) and update on immunization for children aged 0 through 18 years. [cited 2022Nov23]. Available from: https://www.indianpediatrics.net/jan2021/jan-44-53.html







