What is Considered as Diabetes Denial?
Diabetes Denial May Lead to Some Complications.
What are Some Ways to Manage Diabetes?
These Habits Can Help Manage Diabetes.
What is Hypoglycaemia?
What are some ways to manage your Hypoglycaemia?
Sanofi_PA_8_Hypoglycaemia_-_Animation_Video_v.7_-_With_2_disclaimers_in_vo_5
The Right Balance with Amaryl®
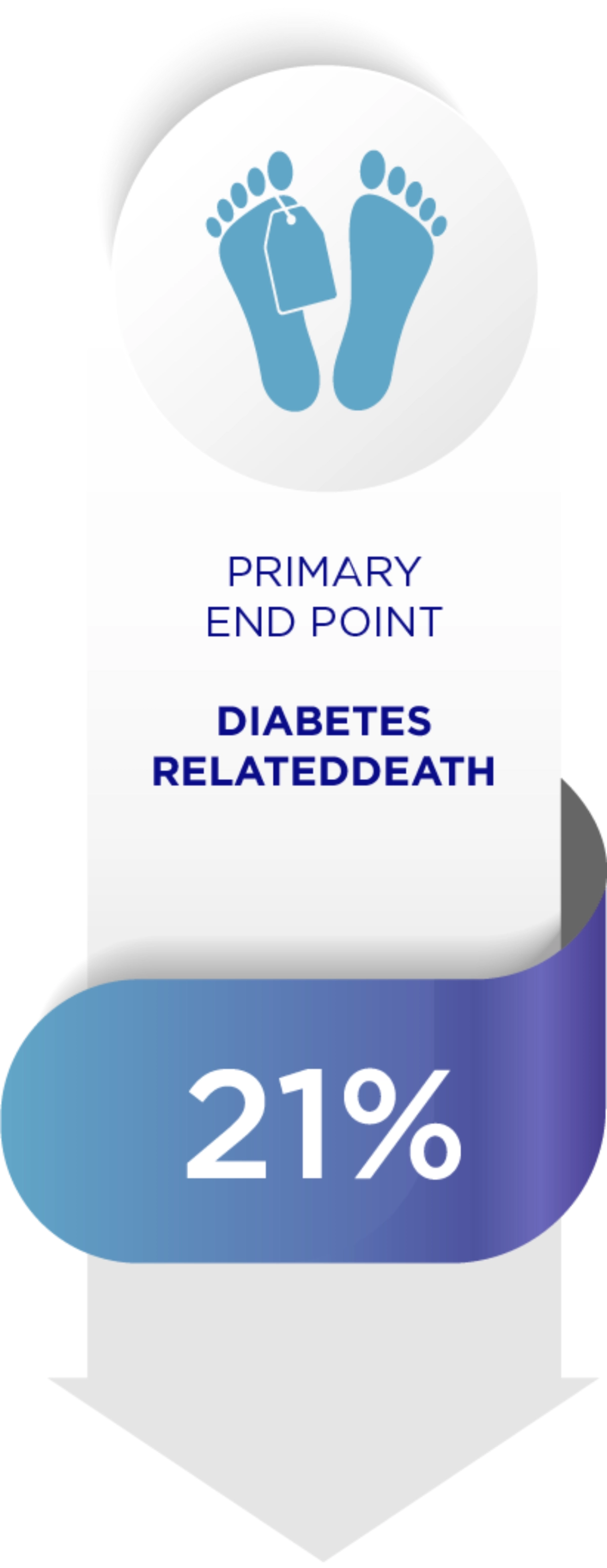
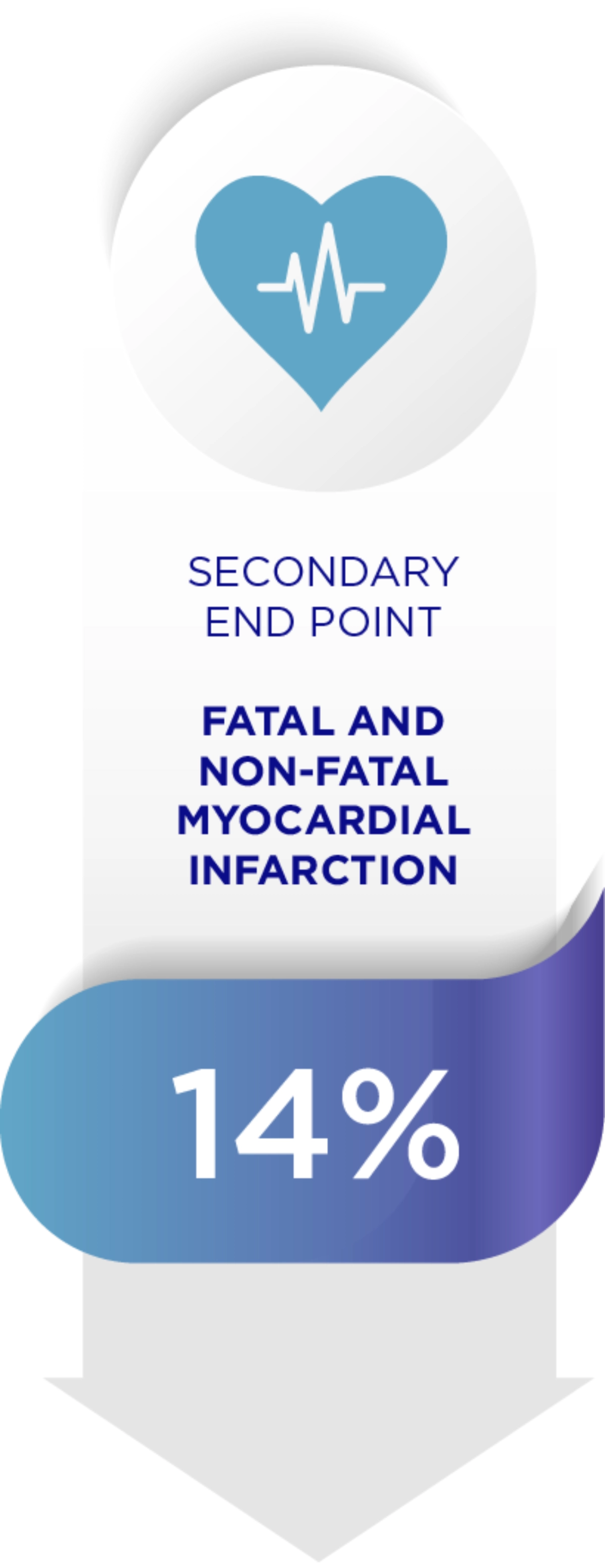
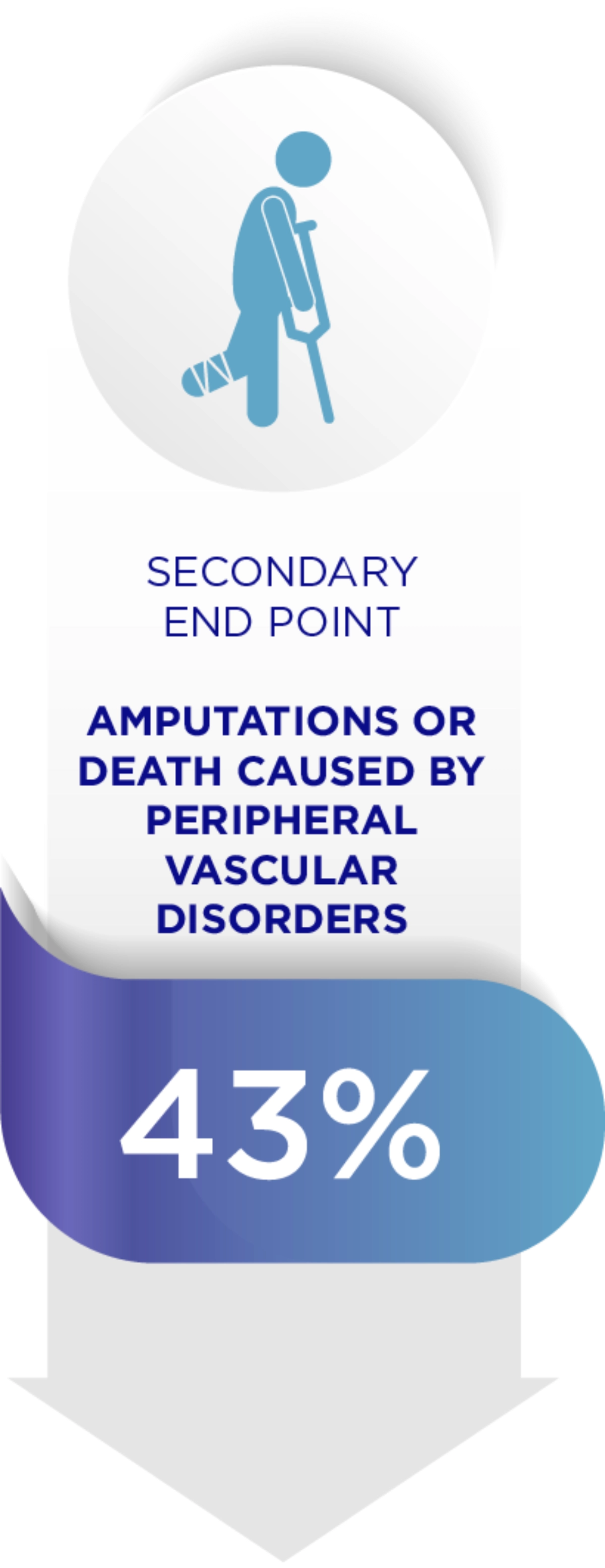
Amaryl can reduce diabetes related complications1
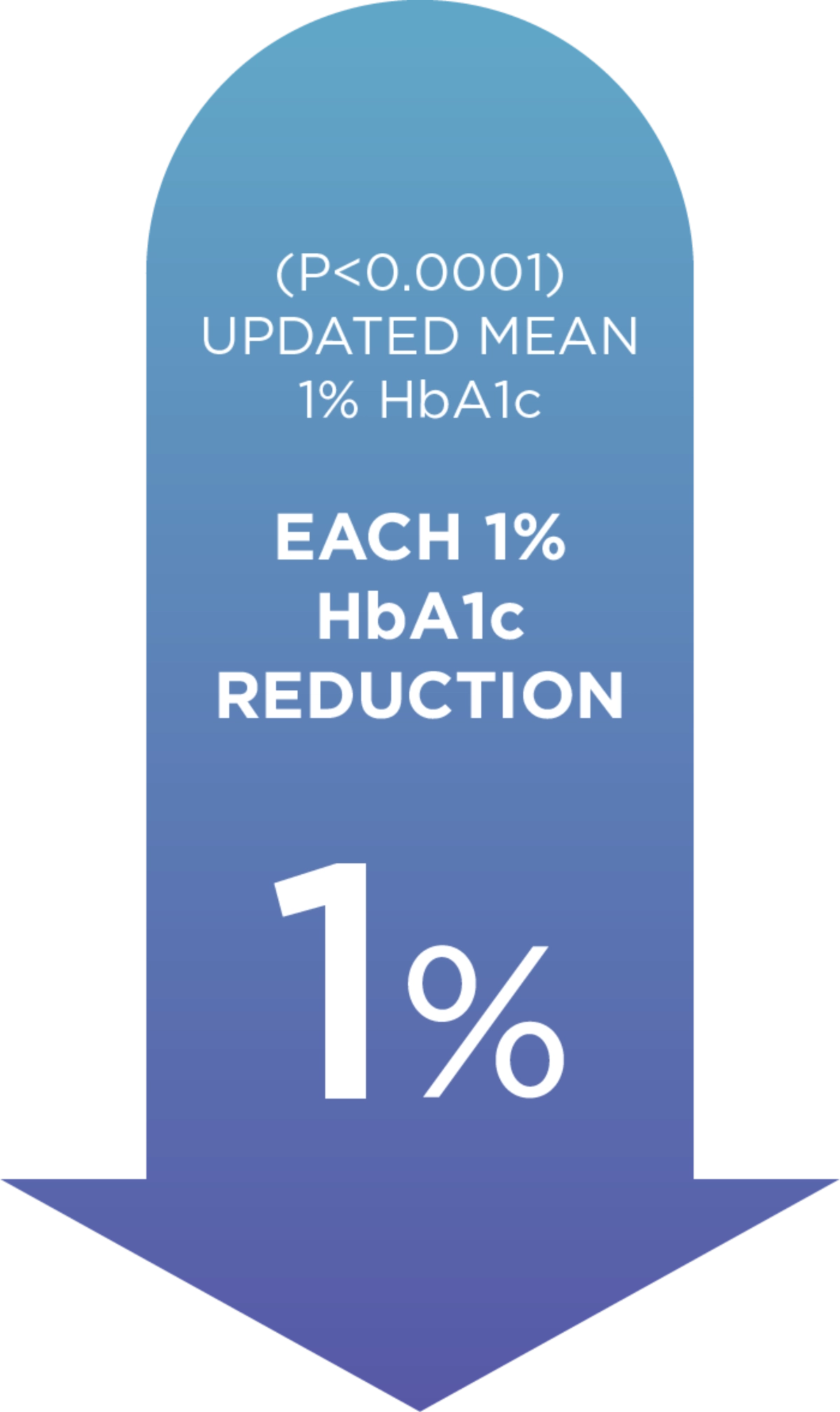
When it comes to Diabetes, each 1% decrease in HbA1c levels reduces complications.1
Adapted from Starton IM et al 2000, BMJ
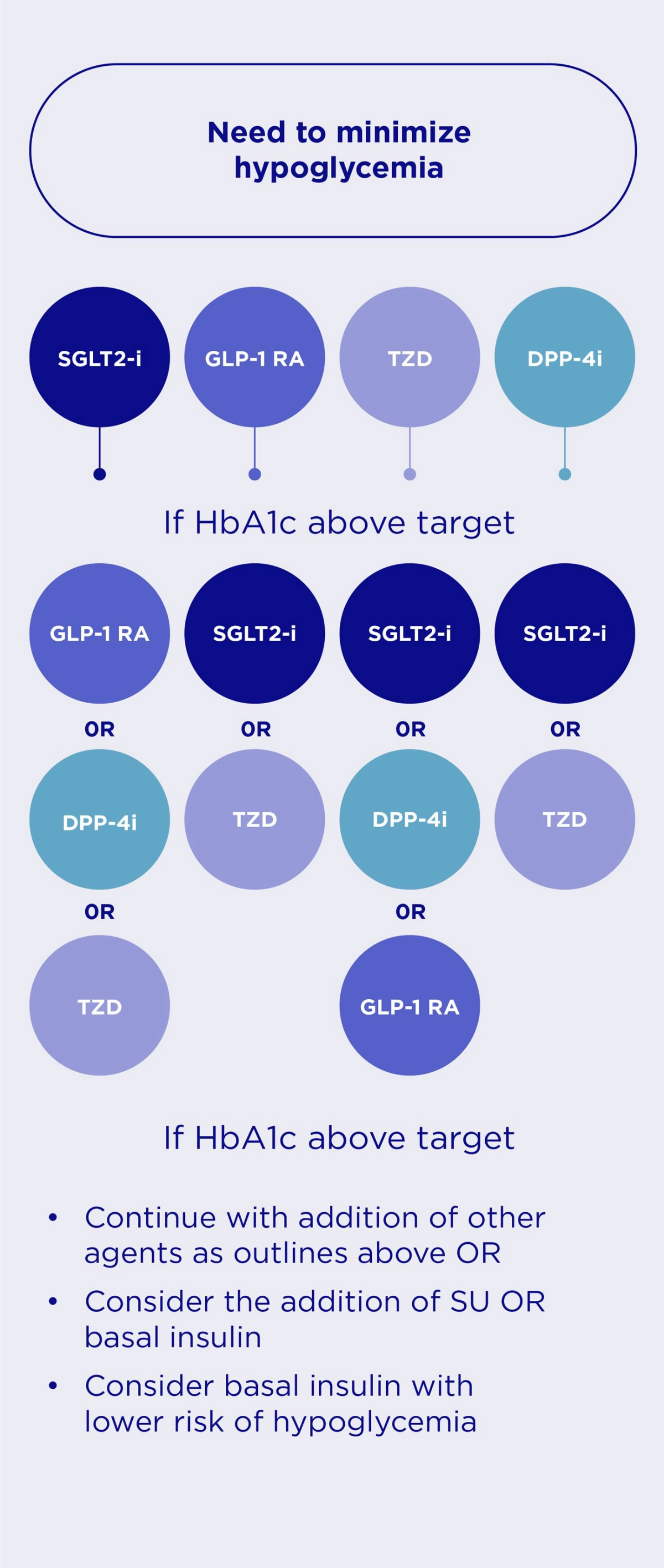
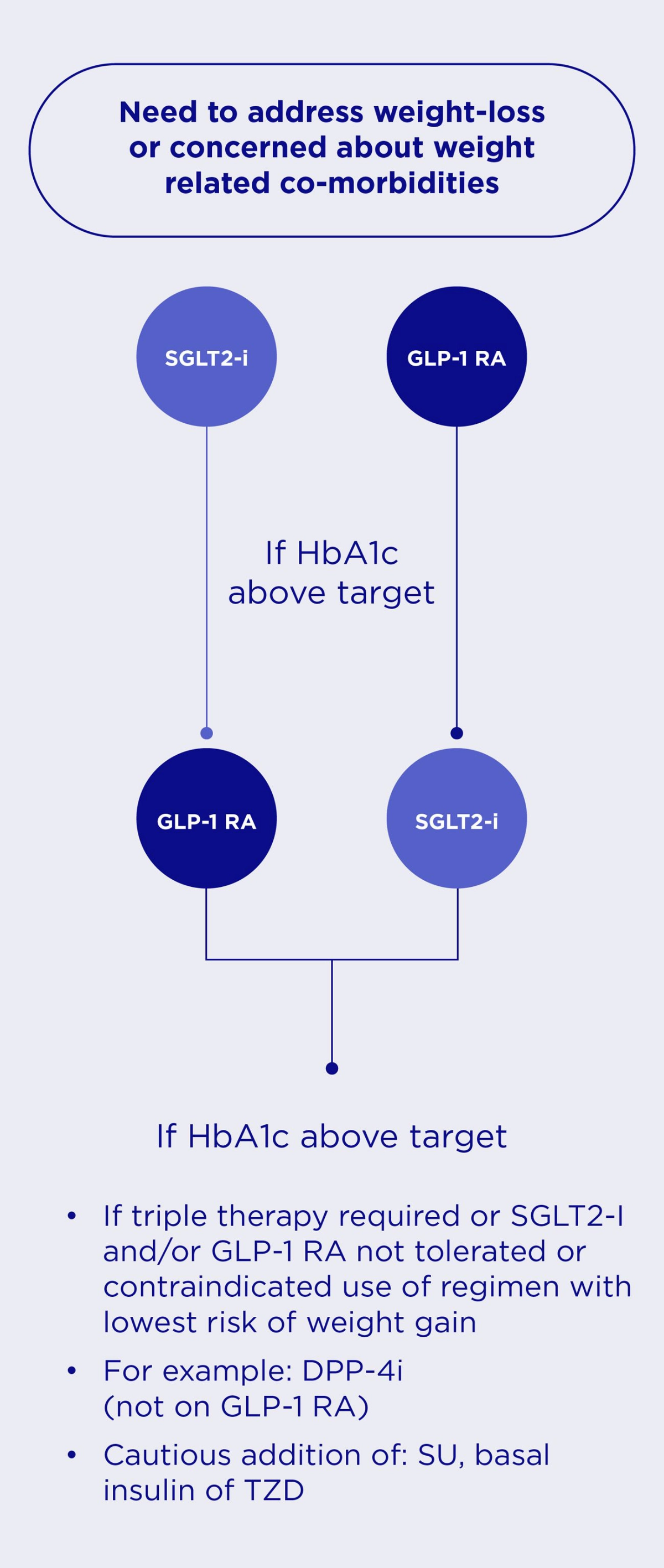
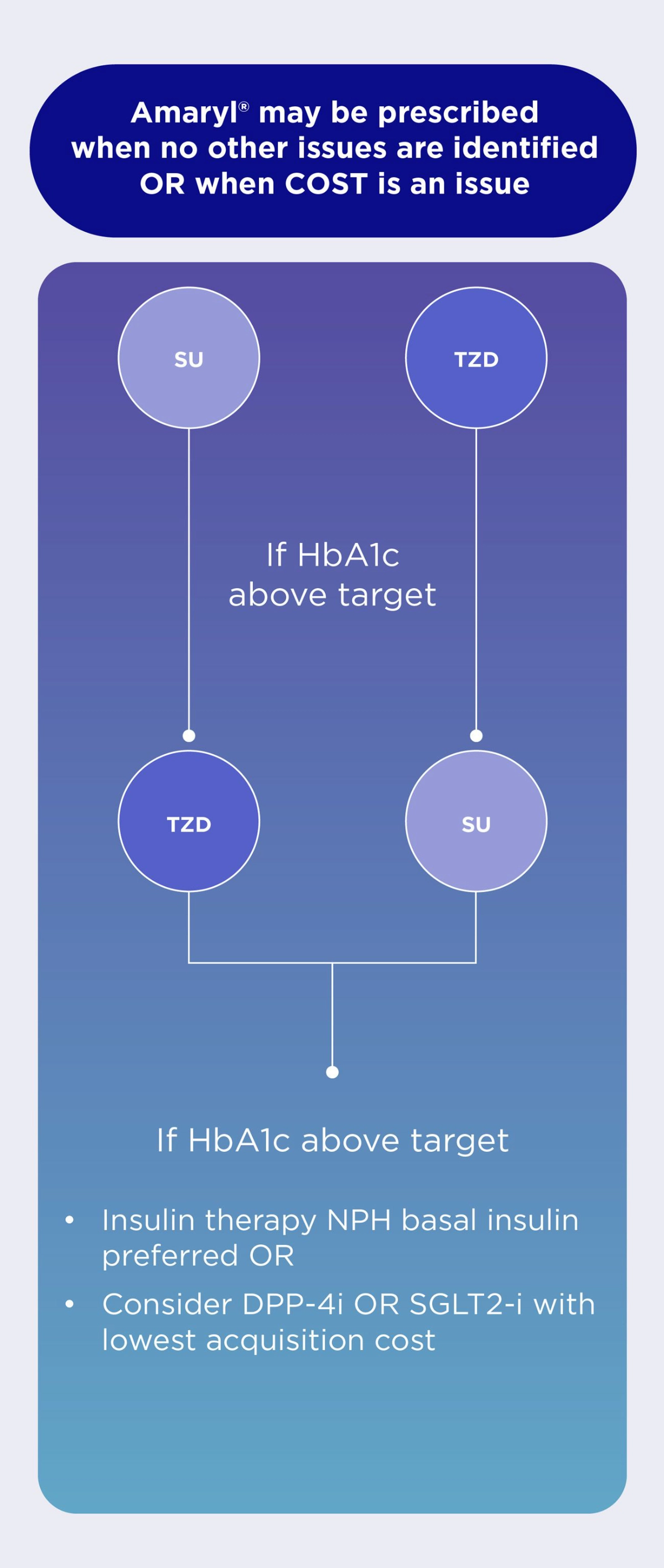
In patients without established ASCVD or HF, international guidelines recommend the following in the management of HbA1c levels.2
Intensification of therapy, with the initiation of additional antidiabetic medication, is often inappropriately delayed. Studies demonstrate that the median time to treatment intensification among those in whom metformin monotherapy failed exceeded 1 year.
People with T2D may be subject to increased glycemic burden for 7.2 years prior to therapy change1
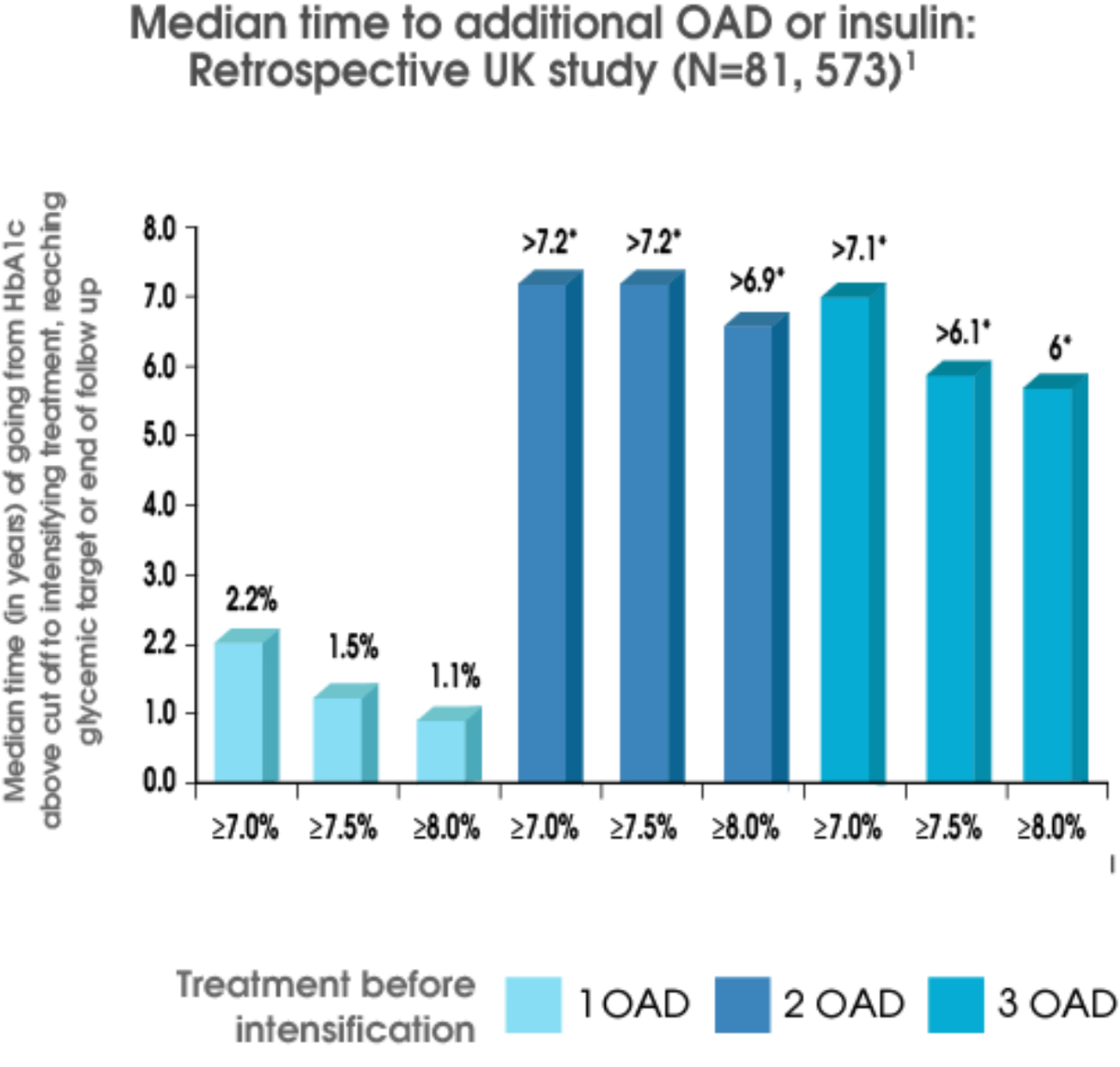
Stepwise treatment intensification remains a common approach to T2D management; however, often results in clinical inertia.2
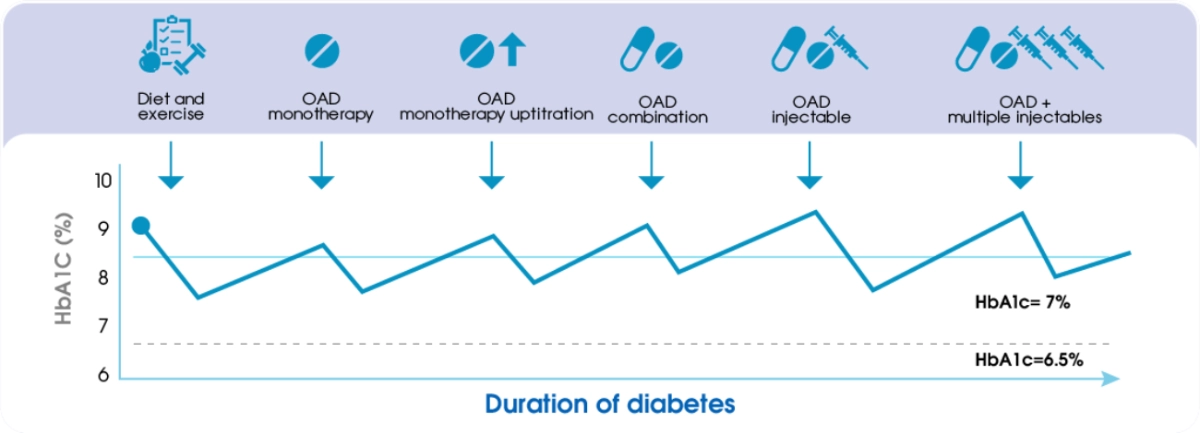
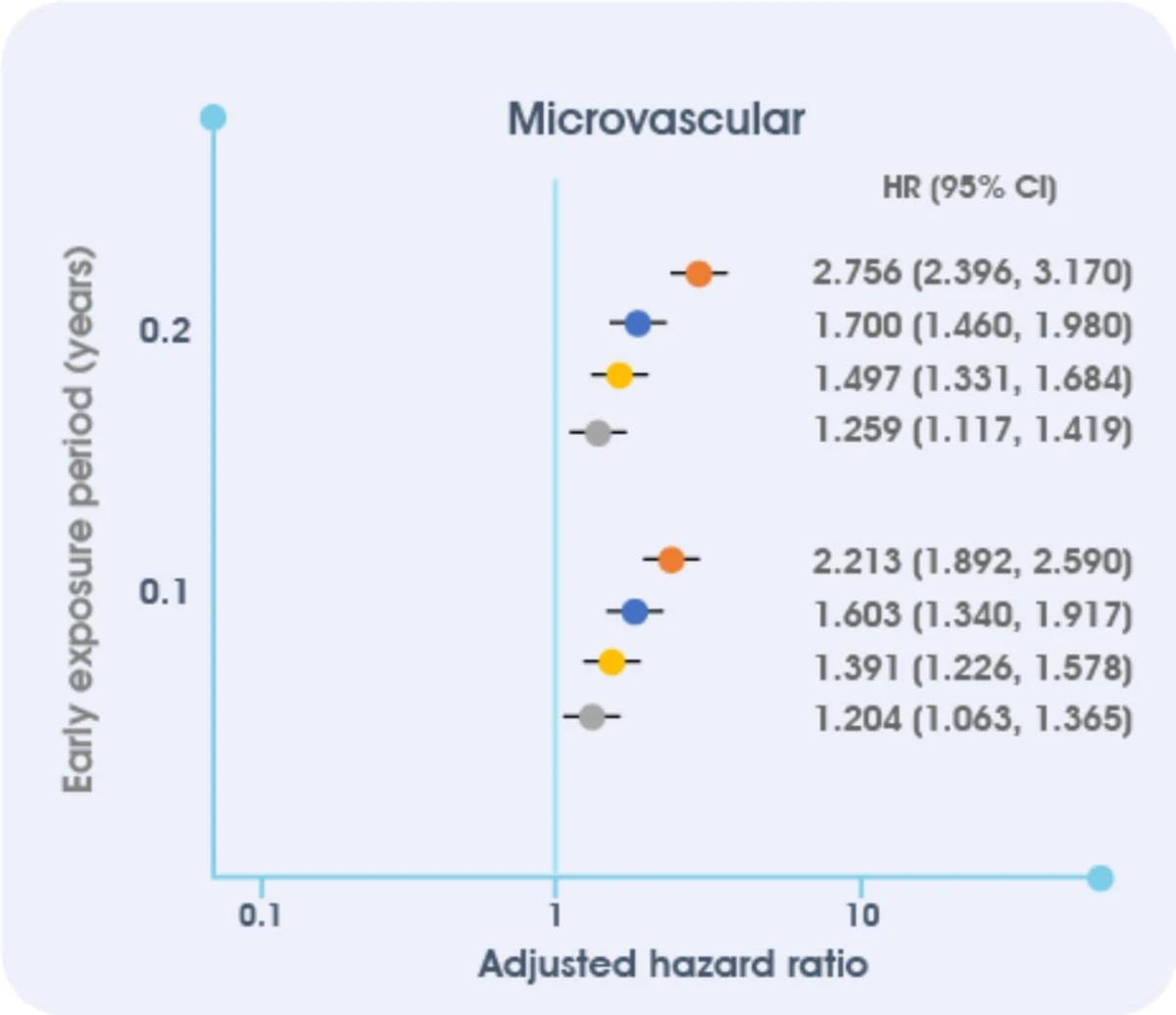
Insufficient glycemic control is associated with the risk of microvascular complications.
Among people with newly diagnosed diabetes and 10 years of survival after diagnosis, HbA1c levels ≥ 6.5% for the first year after diagnosis were associated with worse outcomes3
This cohort study of managed care people with newly diagnosed T2D and 10 years of survival (1997–2013, average follow-up 13.0 years, N = 34,737) examined associations between HbA1c <6.5% (<48 mmol/mol), 6.5% to <7.0% (48 to <53 mmol/mol), 7.0% to <8.0% (53 to <64 mmol/mol), 8.0% to <9.0% (64 to <75 mmol/mol), or 9.0% ( 75 mmol/mol) for various periods of early exposure (0–1, 0–2, 0–3, 0–4, 0–5, 0–6, and 0–7 years) and incident future microvascular (end-stage renal disease, advanced eye disease, amputation) and macrovascular (stroke, heart disease/failure, vascular disease) events and death, adjusting for demographics, risk factors, comorbidities, and later HbA1c. CI, confidence interval; HR, hazard ratio.
- Khunti K, et al. Diabetes Care 2013;36:3411
- Del Prato S, et al. Int J Clin Pract 2005;59:1345–55.
- Laiteerapong N, et al. Diabetes Care 2019;42:416–26.
- Stratton Met al. Association of Glycemia with macrovascular and microvascular complications of T2DM (UKPDS 35): prospective observational study. BMJ Vol 321, 40-5412: 12 Aug 2000.
- Davies MJ, et al. Diabetes Care 2018;41:2669–2701
Amaryl® Dual Mode of Action
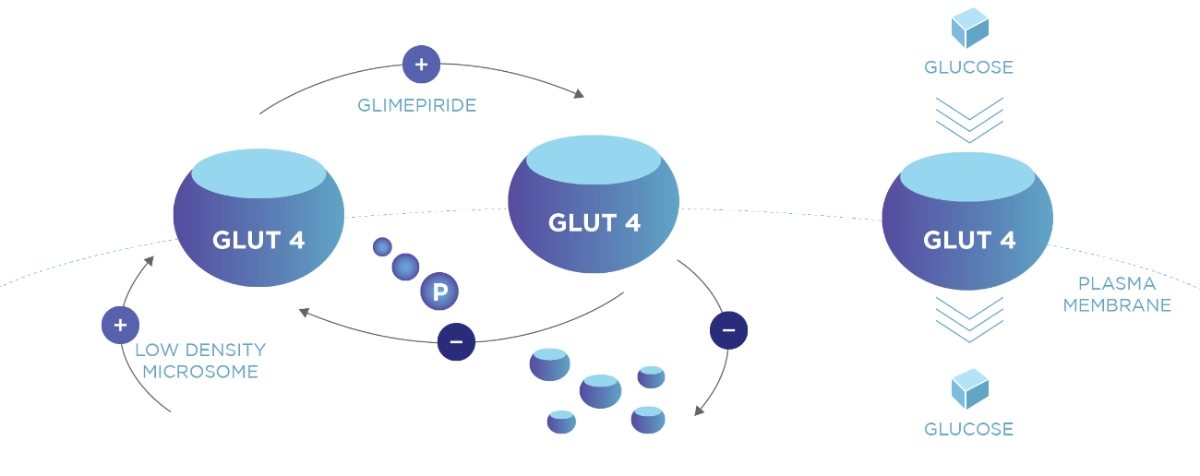
Amaryl has a dual mode of action1 and has extra pancreatic effect on insulin sensitivity.2 The increased GLUT4 expression and Glucose utilization in oxidative muscle, shows evidence of insulin-sensitizing effect of Glimepiride.
Increase insulin sensitivity2
Adapted from Mori RCT et al 2008
Study design
Three-month-old monosodium glutamate {MSG)-induced obese insulin-resistant rats were treated (OG) or not treated (0) with glimepiride for 4 weeks and compared with age-matched non-obese rats (C). Insulin sensitivity were analysed.
Study Objective: The aim ot the present study was to con1irm the Insulin-sensitizer tole of glimeplrlde and to show extra-pancreatic effects of the drug.
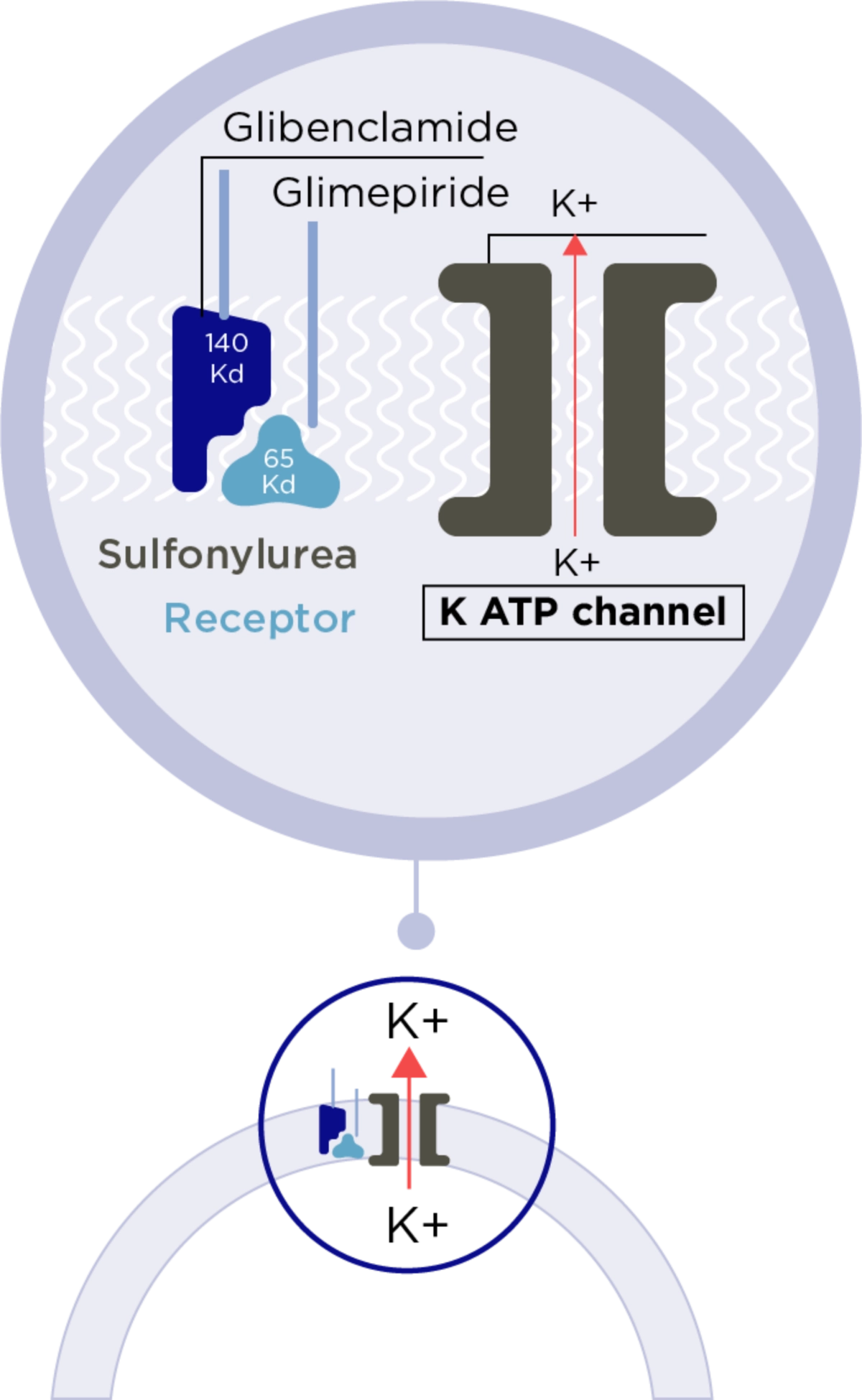
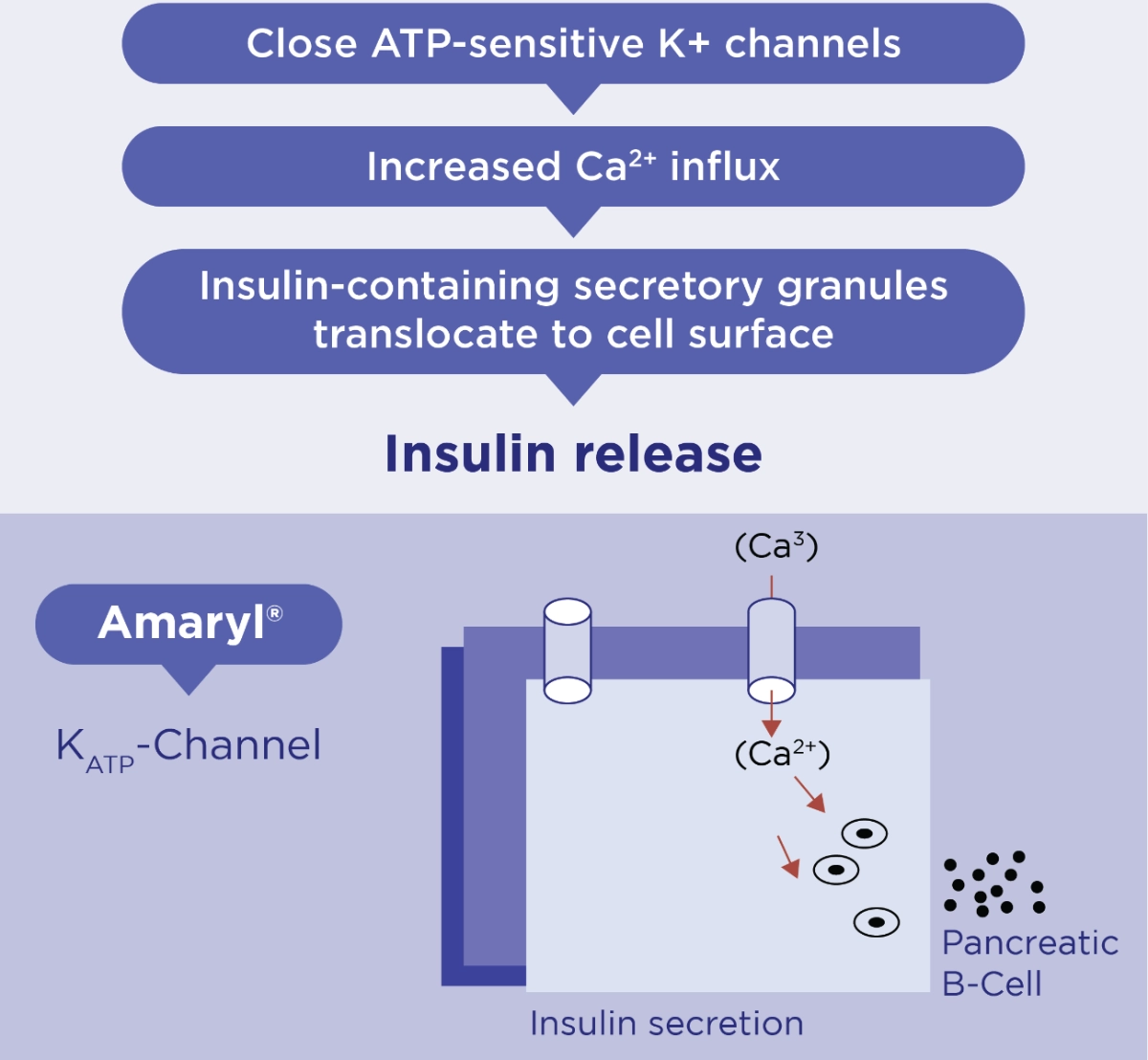
Stimulates Insulin Secretion from B-cells1
Amaryl® binds to SULPHONYLUREA receptors on ß-cells
Amaryl® binds to SULPHONYLUREA receptors on ß-cells
Adapted from Rosak et al 2002. Journal of Diabetes and its complications
The rates of association and dissociation are faster from glimepiride than for glibenclamide so that the duration of receptor binding is much shorter for glimepiride
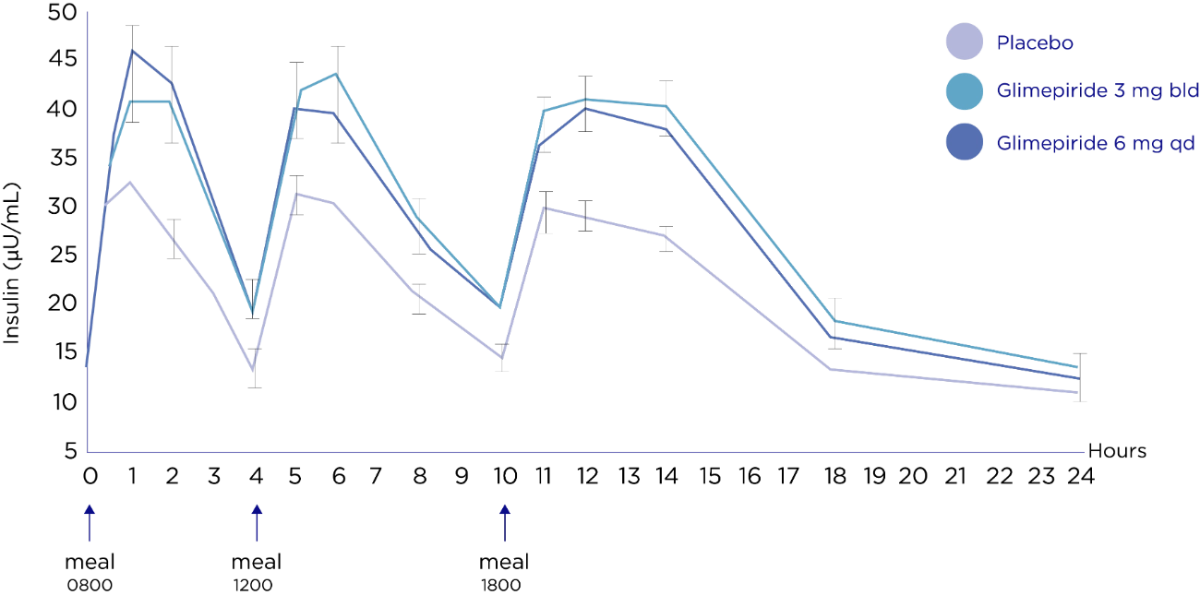
Amaryl® stimulate insulin production primarily after meals, when plasma glucose concentrations are highest, but controls blood glucose throughout the day.*
Insulin is only released in response to meals.3 Both twice and once daily regimens proved equally effective in reducing concentrations of fasting, post breakfast, post lunch and post dinner plasma glucose.3
Study design:
The aim is to investigate the metabolic effects and the frequency of side effects of glimipiride 6mg. 15-week randomized, placebo-controlled, crossover study in 161 patients with T2DM to compare fasting. 24-hours and postprandial plasma insulin and glucose' concentrations. Patients received either glimipiride 3mg twice daily or glimipiride 6mg once daily for 4 weeks. After a 3-week placebo washout period, once-daily and twice-daily regimens were crossed over for a further 4 weeks.
Safety: Two subjects experienced symptomatic hyperglycemia, one during treatment with glimepiride 3 mg once-daily (phase I) and one during a placebo-washout phase. No subject experienced confirmed hypoglycemia. There were no deaths during the study or within 14 days of its completion.
*Only Amaryl 1,2,3,4 Mg are available
- Rosak C. The pathophysiologic basis of ecacy and clinical experience with the new oral antidiabetic agents. Journal of Diabetes and its complications. 16(2012)0231-32.
- Mori R. C.T et al. Glimepiride as insulin sensitizer: increased liver and muscle responses to insulin. Diabetes. Obesity and Metabolism. 602-10:593, 2008.
- Sonnenberg GE, et al. Ann Pharmacother 1997; 31 (6): 671
Amaryl® Efficacy as a monotherapy vs combination therapy
Amaryl Efficacy is Proven in Monotherapy1
Amaryl has proven efficacy as monotherapy by achieving tight glycemic control (HbA1c ≤ 7.2%) in 69% of Glimepiride patients and 32% of placebo patients.1
Amaryl decreased FPG by 46mg/dL more and 2 hour PPG by 86 mg/dL more than placebo (p<0.001)1
Long term efficacy
Amaryl maintained effective glycaemic control throughout 1.5 years in patients with Type 2 diabetes. HbA1c values were continuously lower throughout the 1.5 years.2
SUs have lower rate of treatment failure vs DPP-4i in the first and second year of treatment.2
Combination therapy with Amaryl® is more effective to monotherapy.
Prospective open-label, randomized, comparative, multicenter study, 12 weeks, 305 T2DM patients naïve or uncontrolled on Metformin.3
Adapted from Devarajan T.V. et al Indian J Endocrinol Metab. 2017
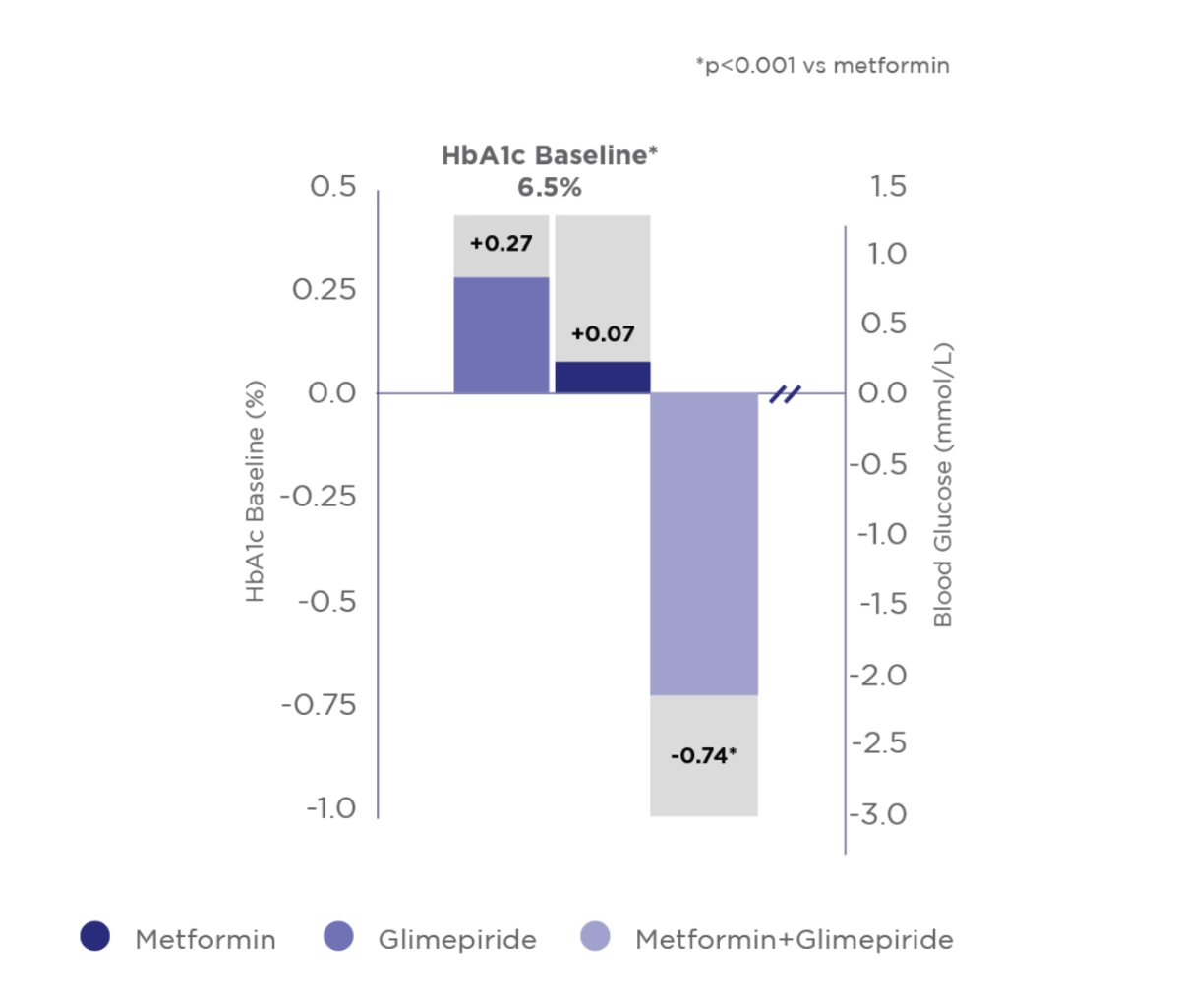
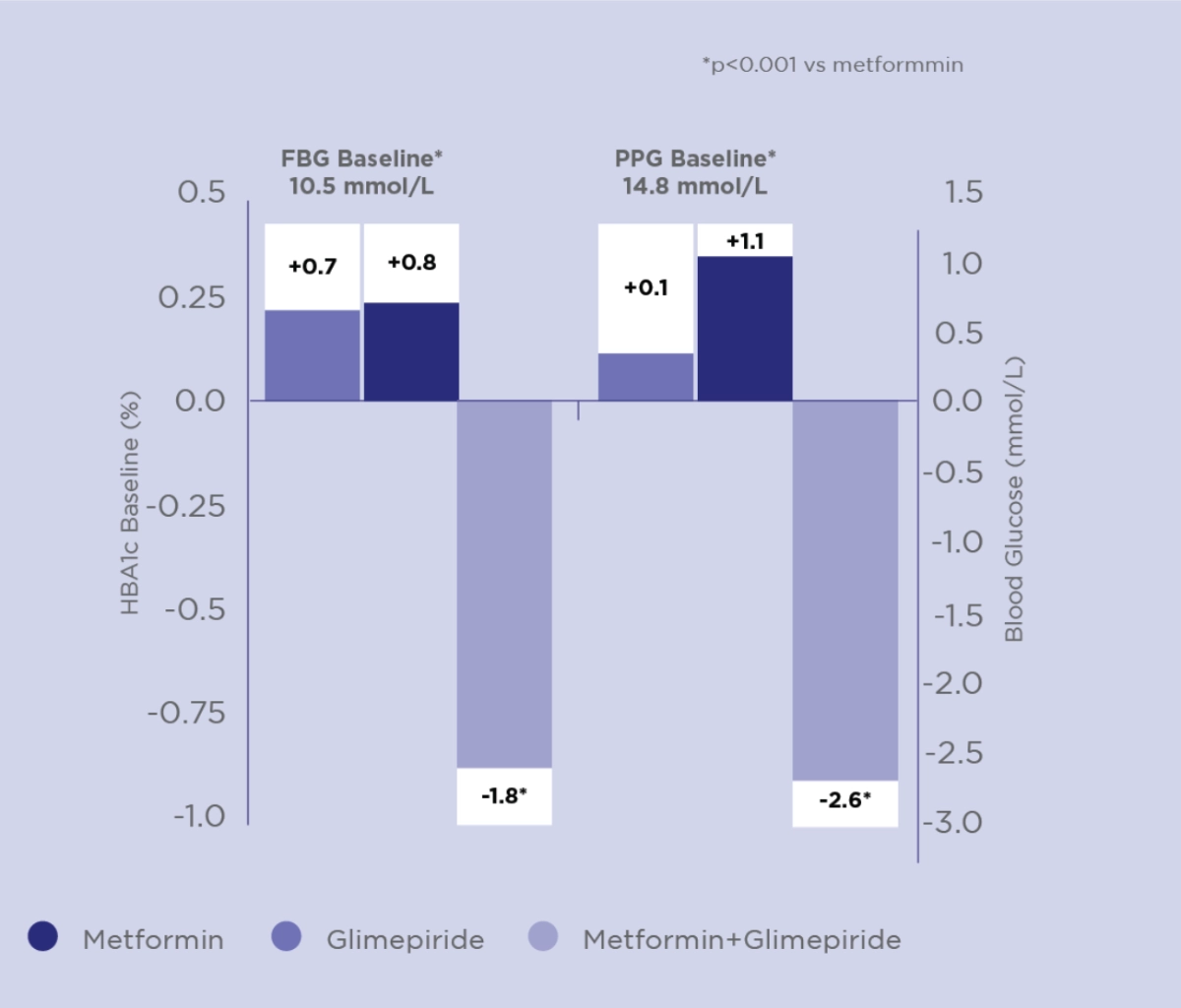
Amaryl® + Metformin Combination has better Glycaemic Control than Monotherapy4
Combination therapy was more e cient in reducing HbA1c, FBG and PPG than Metformin or Amaryl® alone
Change from baseline to week 20 in HbA1c (%), FBG (mmol/L) and PPG (mmol/L)
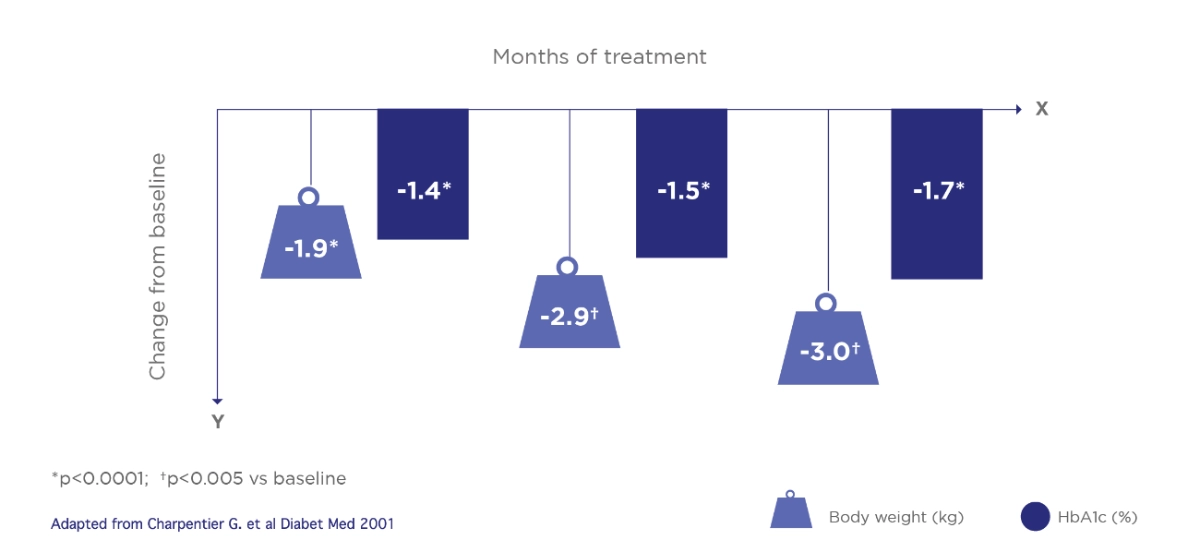
Adapted from Charpentier G. et al Diabet Med 2001
Study design:
To compare the effect of glimepiride in combination with Metformin and the effect of monotherapy in Type 2 diabetic patients. Prospective, multicenter, randomized, doubleblind, double-dummy parallel group study of 372 Type 2 Diabetes Mellitus patients inadequately controlled by Metformin 850 mg tid. Patients received Metformin, Glimepiride (active or placebo) was initiated at a daily dose of 1 mg and increased in a stepwise manner to 2, 4 or 6 mg once daily. Concomitant treatment with any anti-hypertensive or lipid-lowering therapy was permitted provided that dosage remained constant during the study.
RCTs: Random Clinical Trials | SU: Sulfonylureas | All figures are mean values
*For Glimepiride group
Safety: The incidence of symptomatic hypoglycemia was higher in the combination group than in either monotherapy group (P=0.039)
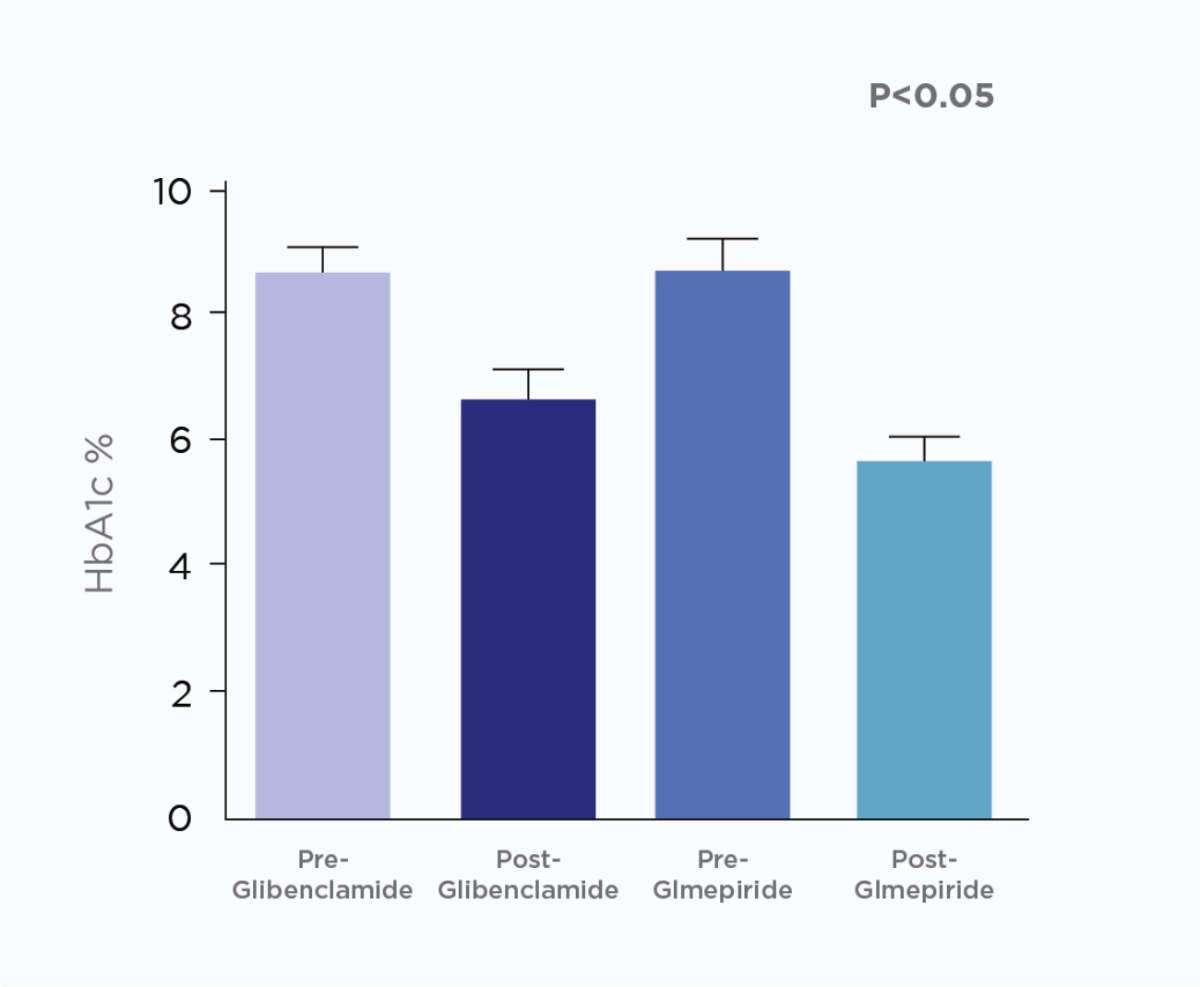
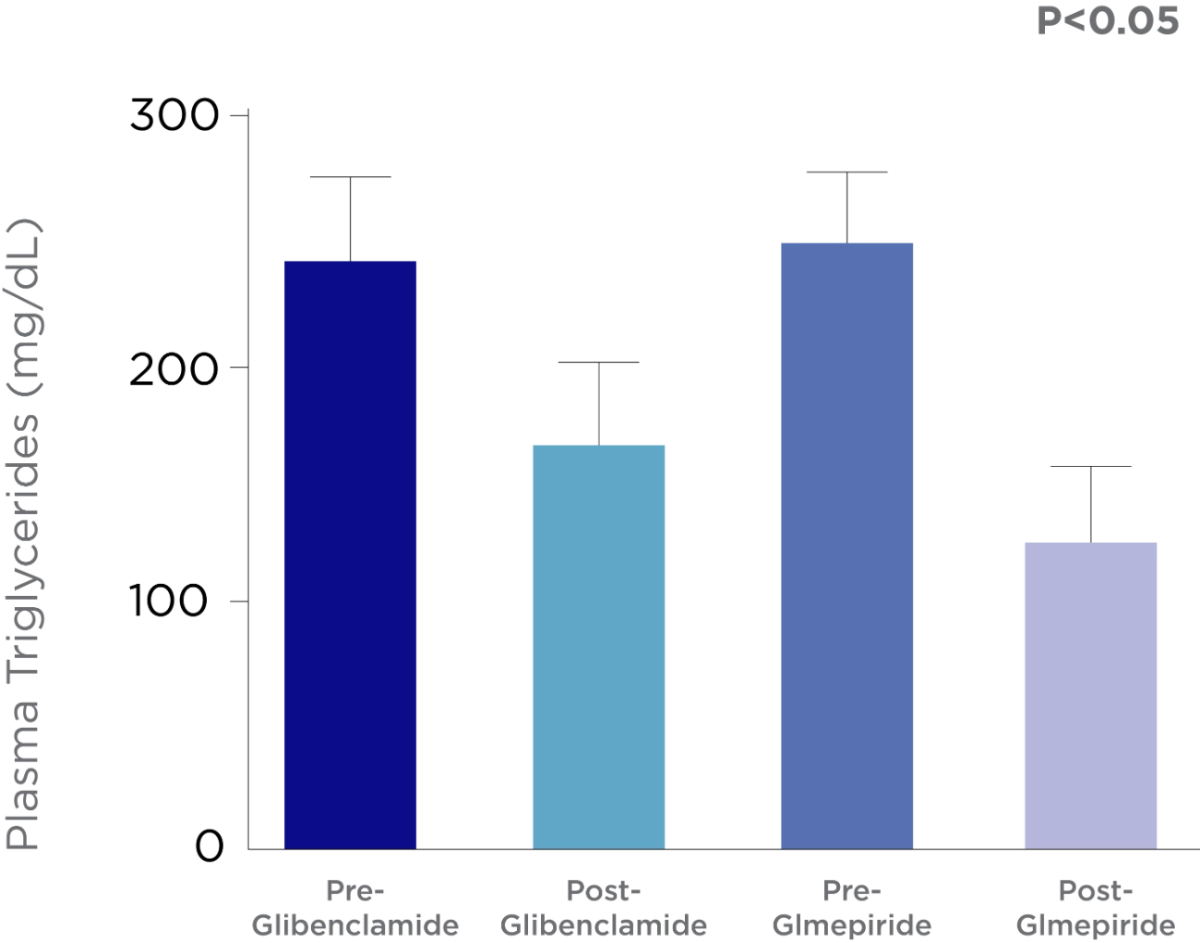
Amaryl® efficacy versus Glibenclamide
Glimepiride provide more potent glycemic control and better lipid profile compared to Glibenclamide in type 2 diabetic patients.5
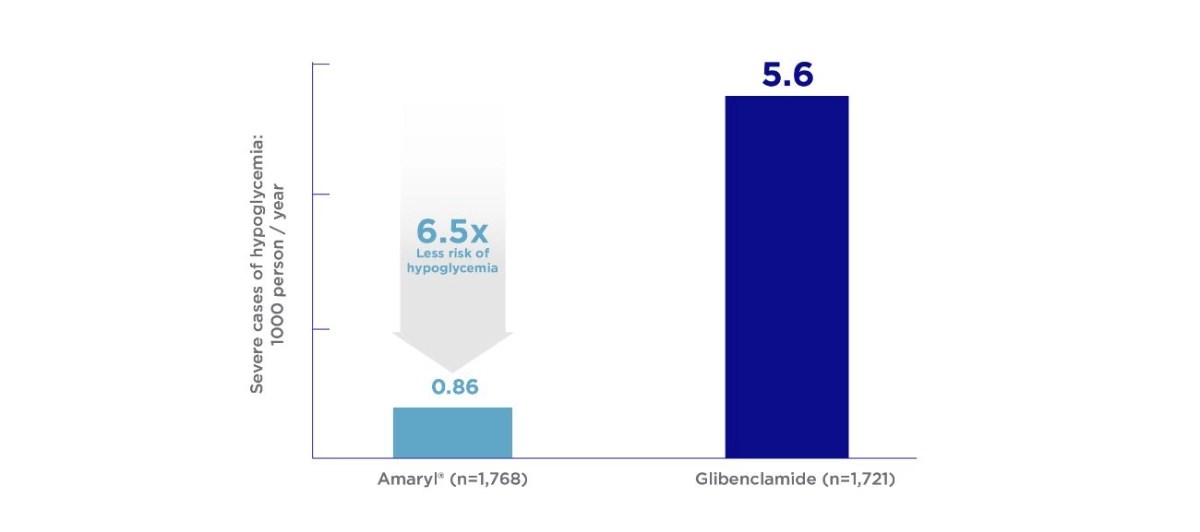
Glimepiride is associated with fewer episodes of severe hypoglycemia than Glibenclamide in routine clinical use.6
Switch & Initiation to a Newer Generation of Sulfonylurea
Glimepiride reduces both fasting plasma glucose and postprandial plasma glucose levels. It can also lower HbA1c values and improve lipid profile compared to other second-generation SUs.5
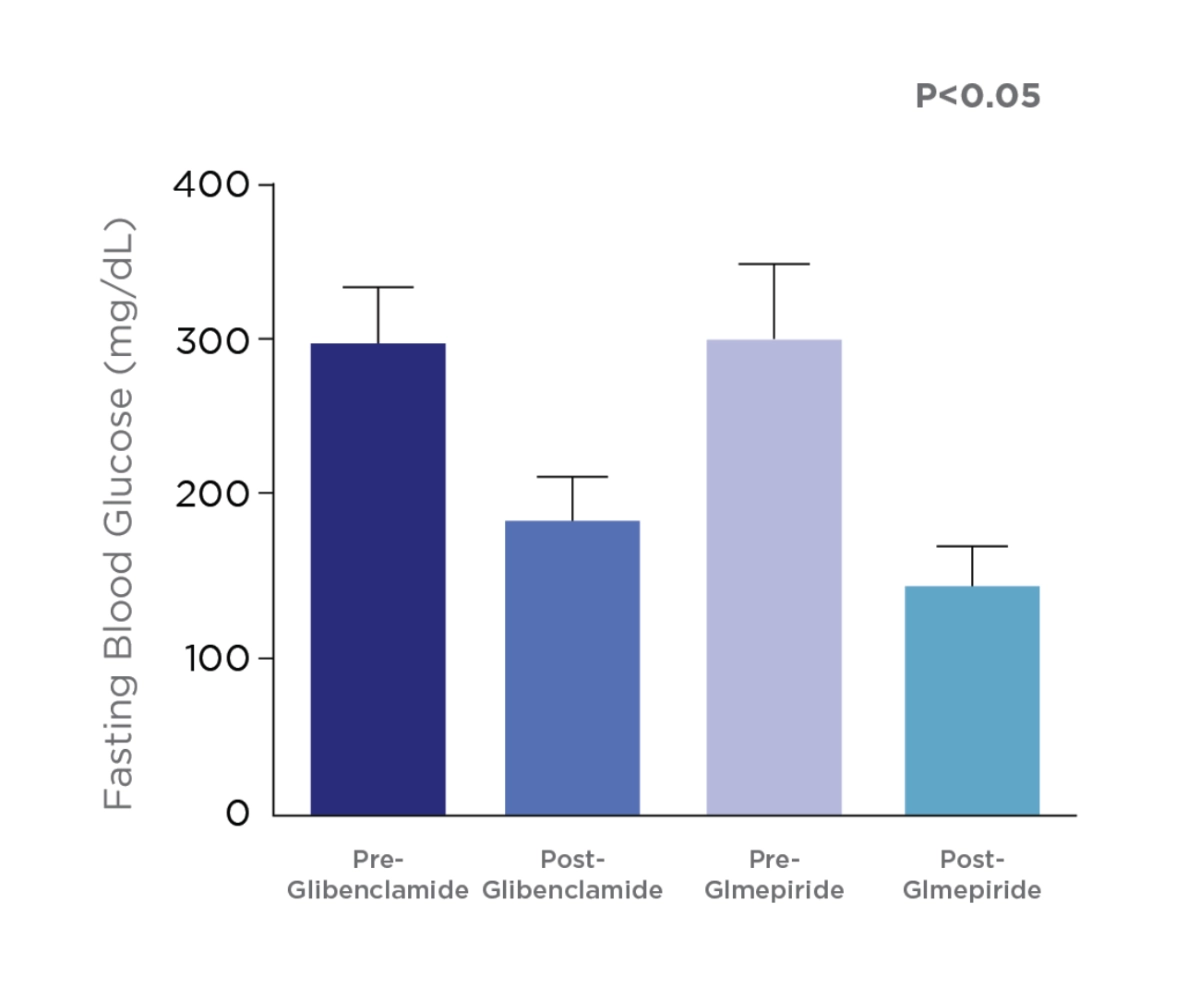
Comparison between the e ect Glmepiride and Glibenclamide on fasting blood glucose & glycosylated hemglobin (HbA1c).
Study Objective:
This study was designed to evaluate the outcome of using glimepiride and glibenclamide in type 2 diabetic patients.
Study Design:
A single blinded randomized clinical trial was adopted, in which 64 already diagnosed diabetic patients (regardless disease duration) were recruited from Al-Yarmouk hospital, and randomized into two groups; 1st group (32 patients) treated with 5 mg glibenclamide, and 2nd group (32 patients) treated with 3 mg glimepride for 4 months.
Adapted from Al-Hamdani, F, 2013
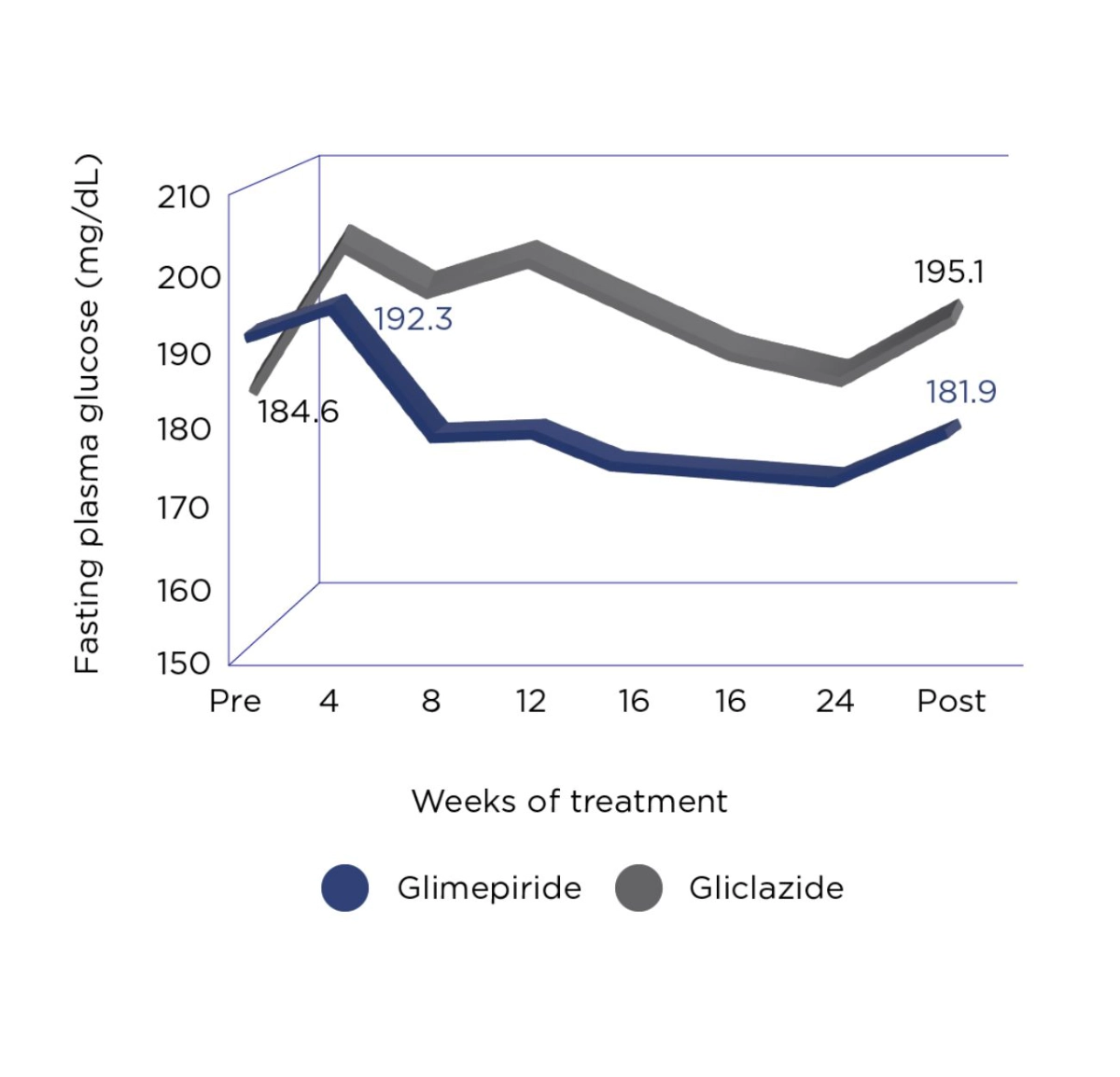
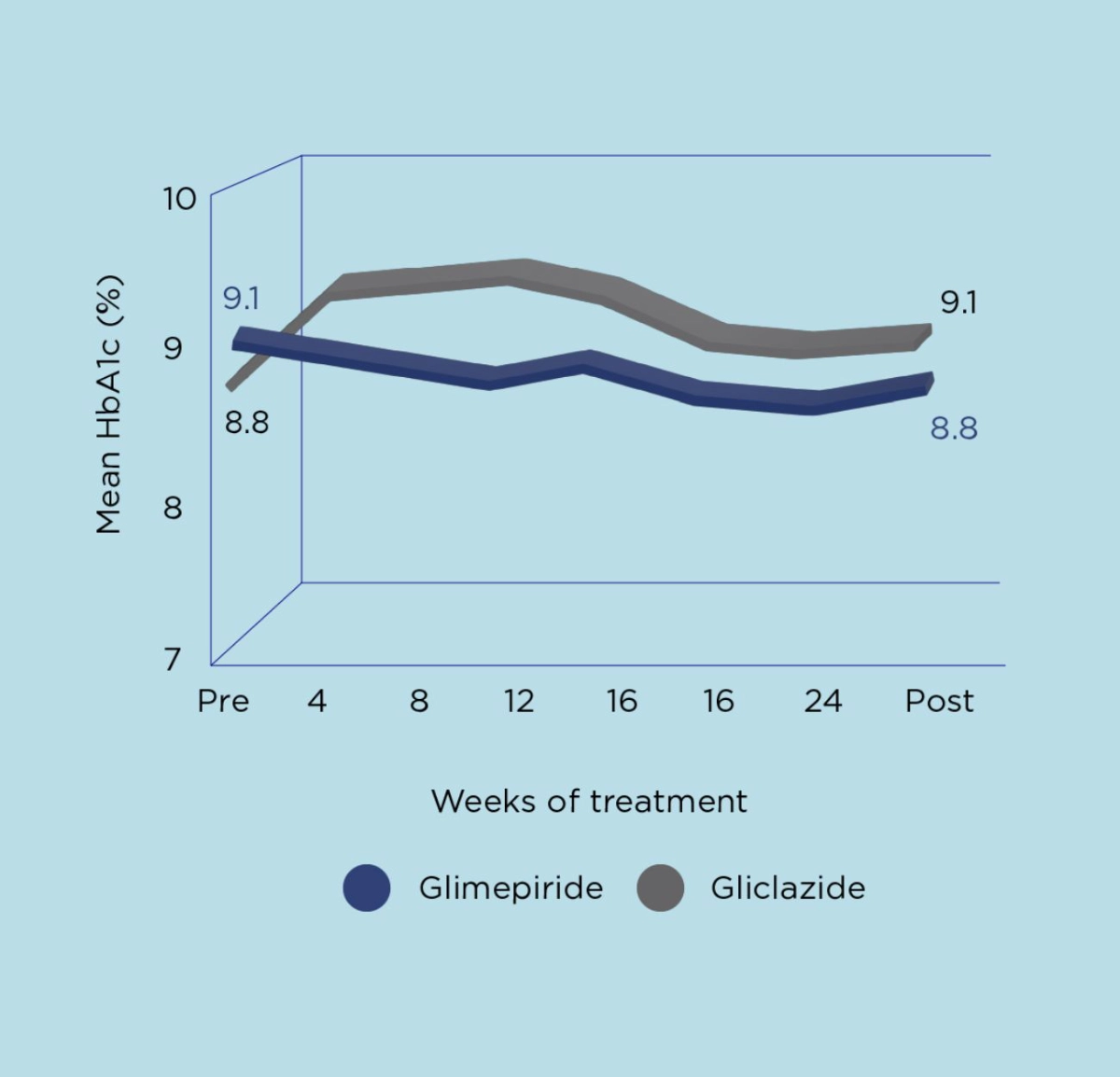
Amaryl® is as effective as Gliciazide and has lower risk of hypoglycemia than Gliclazide.2
In a double-blind comparative study versus gliclazide, glimepiride was as efficacious as gliclazide. However, a subgroup analysis suggested glimepiride may have been effective in severe patients treated by large doses of glibenclamide (6.25 – 10 mg).2
Adapted from Tsumura K. Diabetes Research and Clinical Practice 1995.
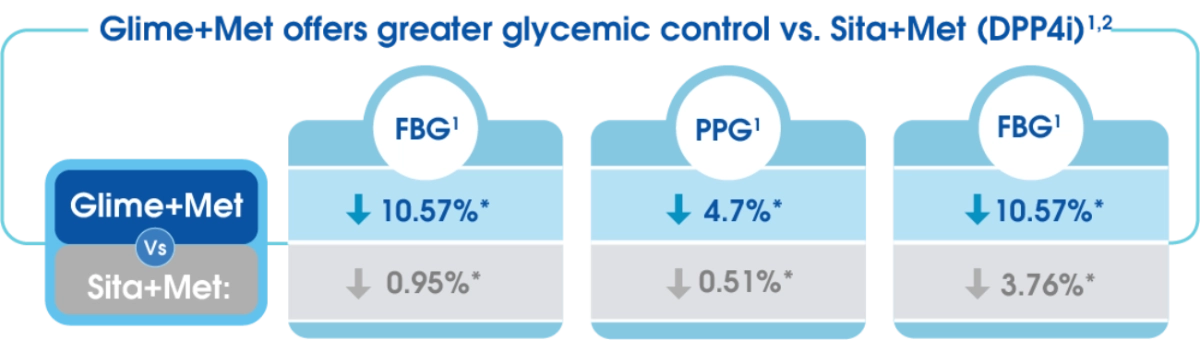
- Amaryl/metformin combination is more effective than Sitagliptin/Metformin combination.3
- SUs has lower rate of treatment failure versus DPP-4i in the first and second year of treatment.9
- Amaryl demonstrated non-inferiority for 3p MACE vs lingliptin in participants with relatively early T2D and increased CV risk.8
- Schade OS, et al. Placebo-controlled, randomized study of glimepiride in patients with type 2 diabetes mellitus for whom diet therapy is unsuccessful. J Clin Pharmacol 51-38:636;1998.
- Tsumura K. Diabetes Research and Clinical Practice1995;28 S147 -149.
- Devarajan, T. et al. Comparative Evaluation of Safety and E_cacy of Glimepiride and Sitagliptin in Combination with Metformin in Patients with Type 2. Diabetes Mellitus: Indian Multi centric Randomized Trial - START Study. Indian J Endocrinol Metab. 2017 Sep-Oct; 21 (5): 7 45-750.
- Charpentier G, et al. Improved glycaemic control by addition of glimepiride to metformin monotherapy in type 2 diabetic patients Diabet Med 2001; 18 (10):828-34.
- Alhamadani, Fadya & Al-Mefraji, Maitham.M (2013). Comparative Study Between Glimepiride and Glibenclamide in the Treatment of Type 2 Diabetic Patients in Al-Yarmouk Hospital. THE IRAQI POSTGRADUATE MEDICAL JOURNAL. VOL.12.
- Holstein A, Plaschke A, Egberts EH. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev. 2001;17(6):467-473. doi:10.1002/dmrr.235.
- Gonzalez-Ortiz M. et al. E cacy of gllmeplrlde/metformln combination versus gllbenclamlde/ metformln In patients with uncontrolled type 2 diabetes mellttus. Journal of Diabetes and Its Complications 2008; 23: 376-379.
- Rosenstock, J et al. “E ect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial.” JAMA vol. 322,12 (2019): 1155-1166. doi:10.1001/jama.2019.13772.
- Mamza J,et al. Important di erences in the durability of glycaemic response among second-line treatment options when added to metformin in type 2 diabetes: a retrospective cohort study. Ann Med 2016; 48 (4) : 224 -34.
While generics can help decrease healthcare costs, quality and performance of generics should be carefully monitored to ensure patients are receiving the optimal efficacy and safety benefits offered by Amaryl.®
Amaryl® vs. generics
74% Grid items of generics were not of equivalent quality or performance compared with Amaryl®*1
- Higher level of impurities
- Different level of residual solvent
- Dissolution profile not similar
- Lower content of active compound
*17 out of 23 marketed generics forms versus Amaryl®
Start study
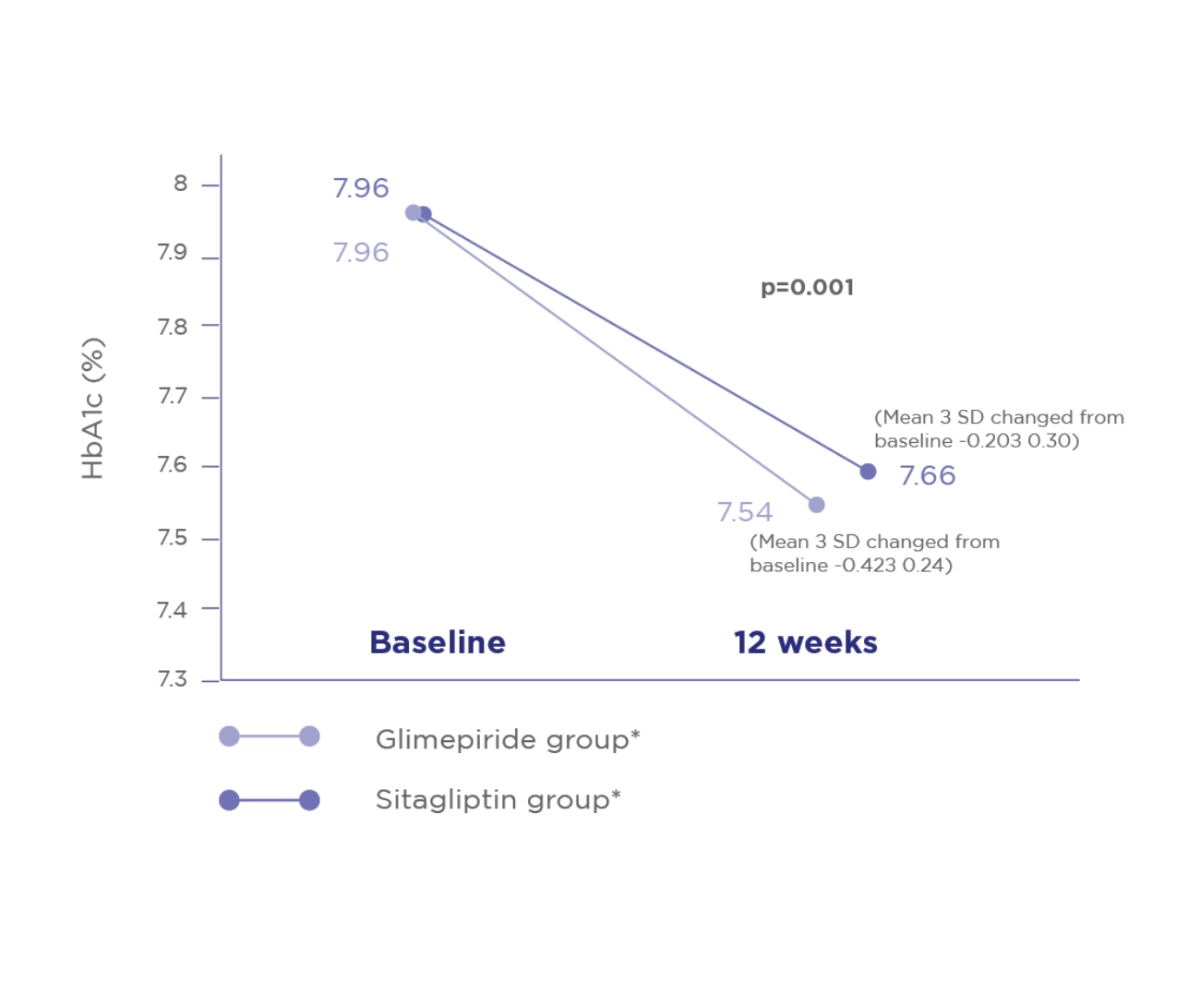
Comparative evaluation of safety and efficacy of Glimepiride and Sitagliptin in combination with Metformin in patients with T2D.2
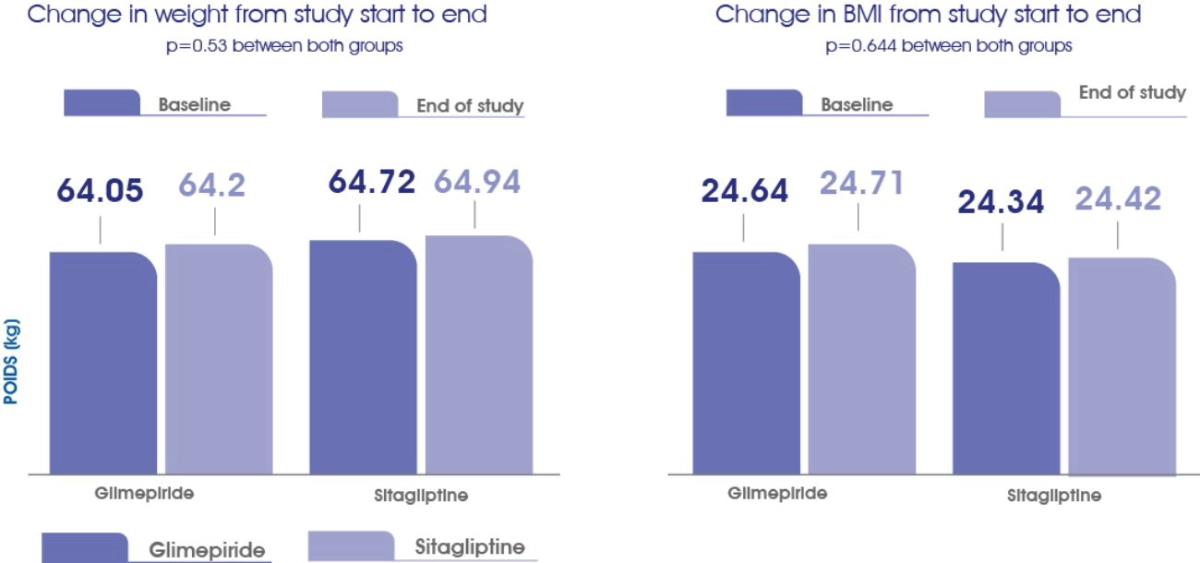
The incidences of hypoglycemic symptoms were comparable in both groups®
The change in body weight and BMI from baseline to the end of study was comparable in both groups®
- Attorrese G, Massi-Benedetti M. [published correction appears in Diabetes Technol Ther. 2007 Oct;9(5):482]. Diabetes Technol Ther. 2007;9(3):287-296.
- Devarajan TV, Venkataraman S, Kandasamy N, et al. Indian J Endocrinol Metab. 2017;21(5):745-750.
The Carolina Trial*
Amaryl® demonstrated non-inferiority for 3p-MACE* vs linagliptin in participants with relatively early T2D and increased CV risk.1
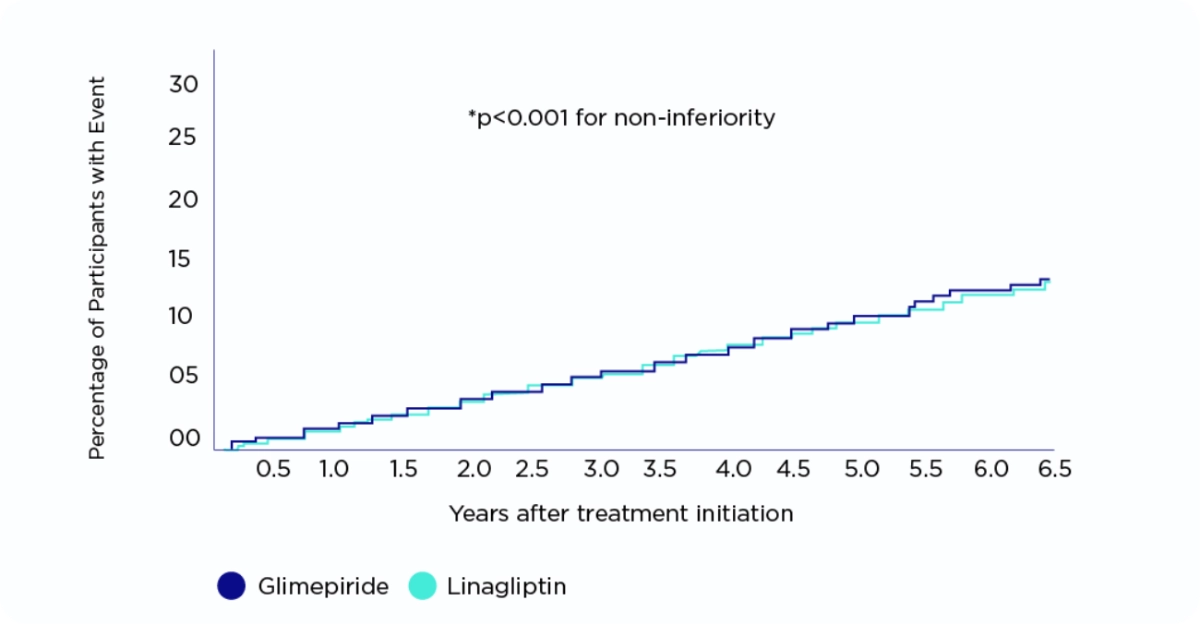
Rosenstock J, JAMA, 2019
CV: Cardiovascular | T2D: Type 2 Diabetes | CAROLINA: Cardiovascular outcome trial of Linagliptin versus Glimepiride in patients with type 2 diabetes | 3P-MACE: 3 points major adverse cardiovascular events.
The study done in :
- 43 countries
- 607 centers
- 6042 patients
Study design:
Randomized, double-blind, active-controlled, non-inferiority trial, with participant screening from November 2010 to December 2012. Adults with type 2 diabetes, glycated hemoglobin of 6.5% to 8.5%, and elevated cardiovascular risk were eligible for inclusion. Glimepiride arm: 3010 patients (1 to 4 mg once daily) Linagliptin arm: 3023 patients (5 mg once daily).
Study objective:
This trial assessed cardiovascular outcomes of linagliptin vs glimepiride (sulfonylurea) in patients with relatively early type 2 diabetes and risk factors for or established atherosclerotic cardiovascular disease.
Primary end point:
The primary outcome was time to first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke with the aim to establish noninferiority of linagliptin vs glimepiride.
Safety data:
At least 2 episode of hypoglycemic adverse events occurred in 320 (10.6%) participants in the linagliptin group and 1132 (37.7% in the glimepiride group (HR, 0.23 [95%CI, 0.21-0.26])
- Rosenstock, J et al. “Eect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial.” JAMA vol. 322,12 (2019): 1155-1166. doi:10.1001/jama.2019.13772.
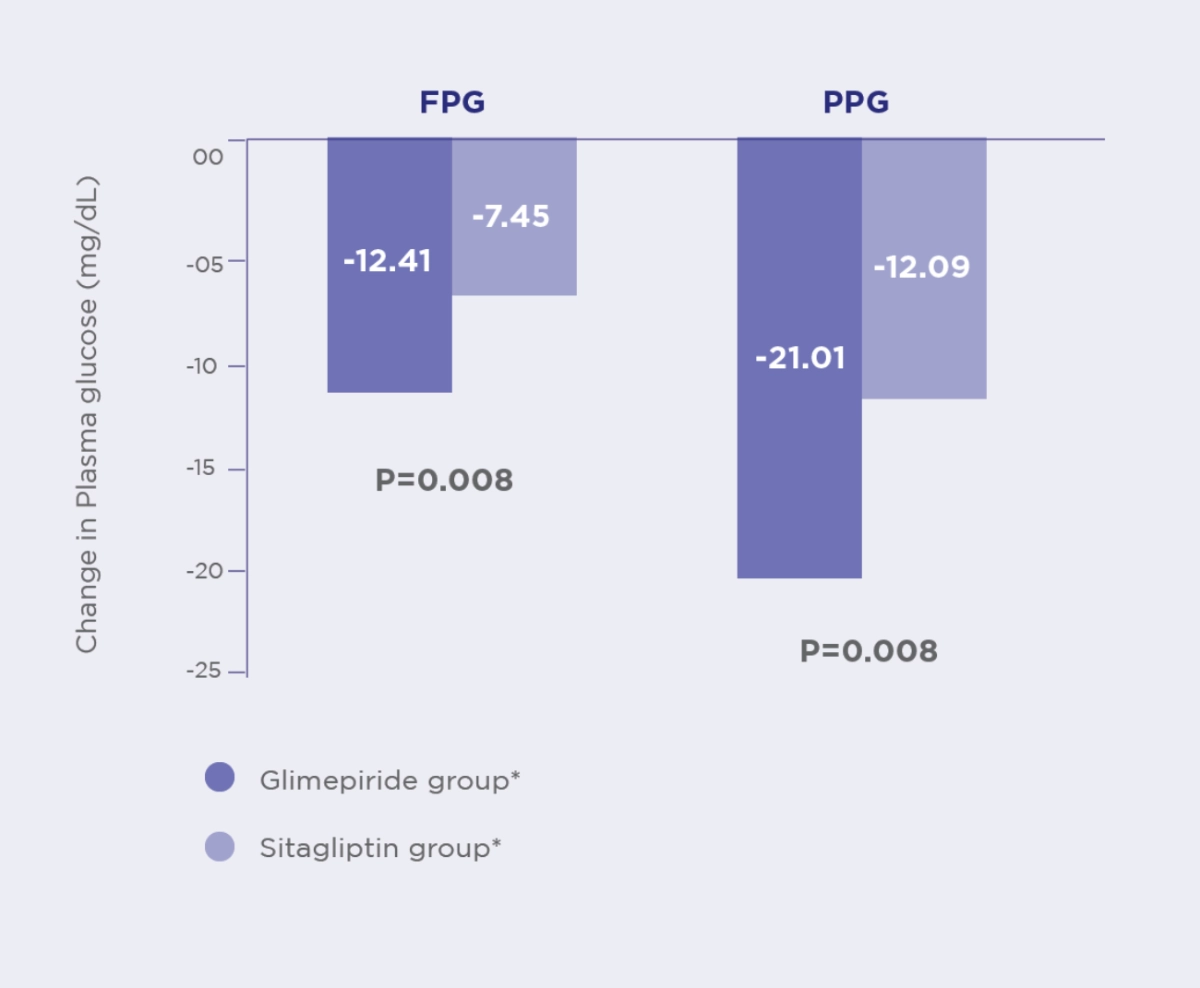
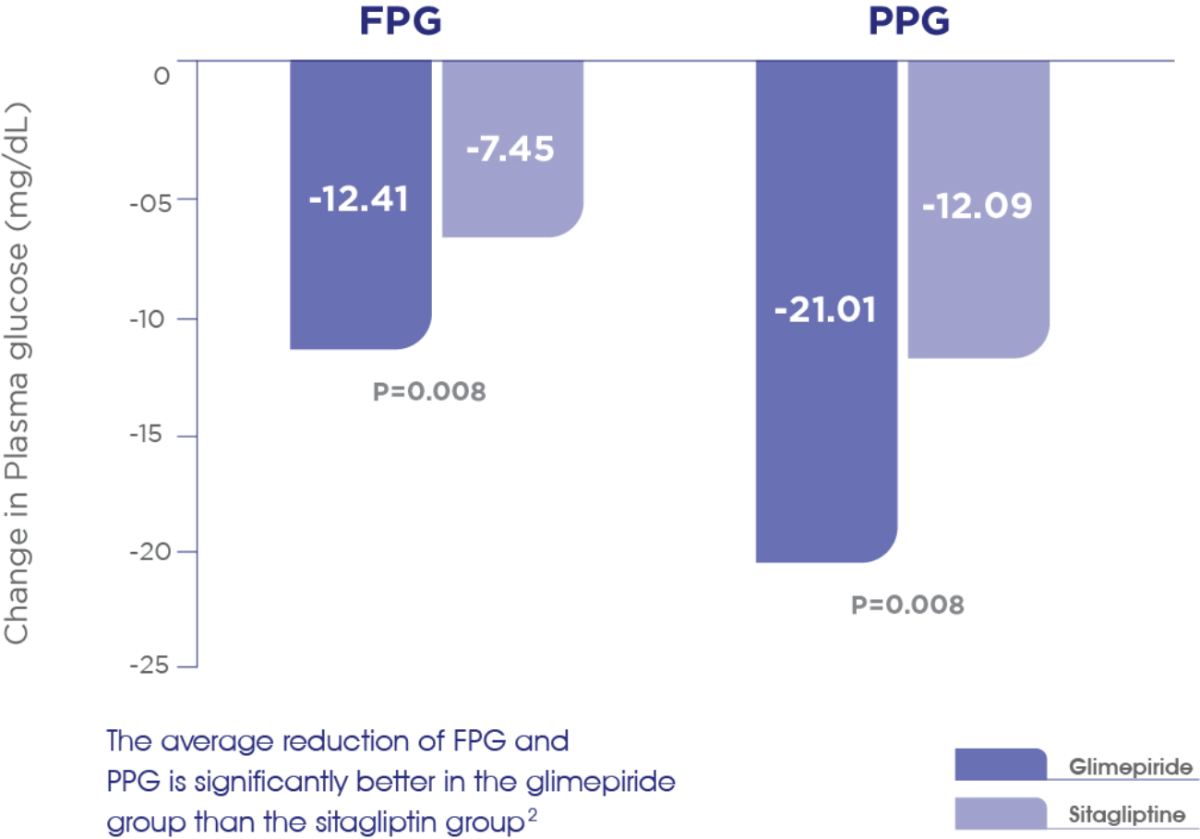
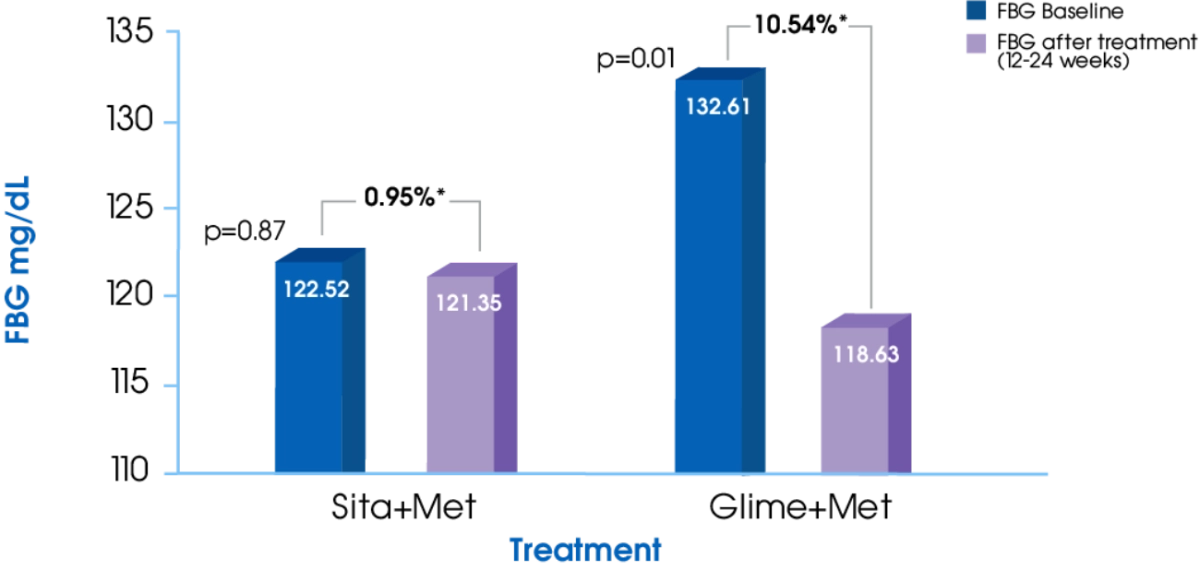
FBG1
Glime+Met offers significantly greater reduction in FBG compared to Sita+Met1
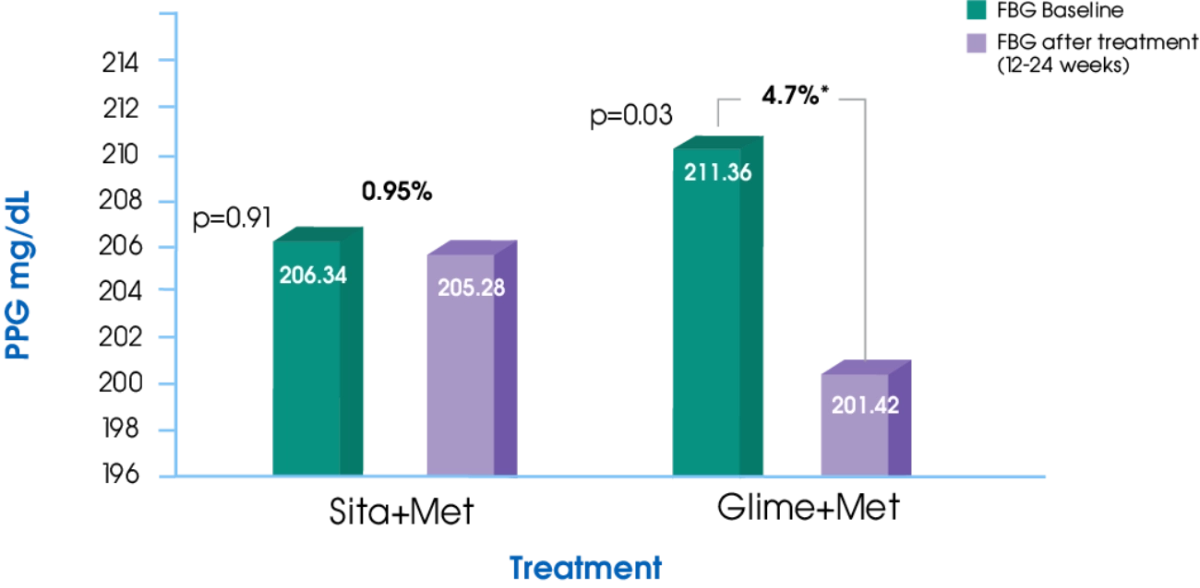
PPG1
Glime+Met offers significantly greater reduction in PPG compared to Sita+Met1
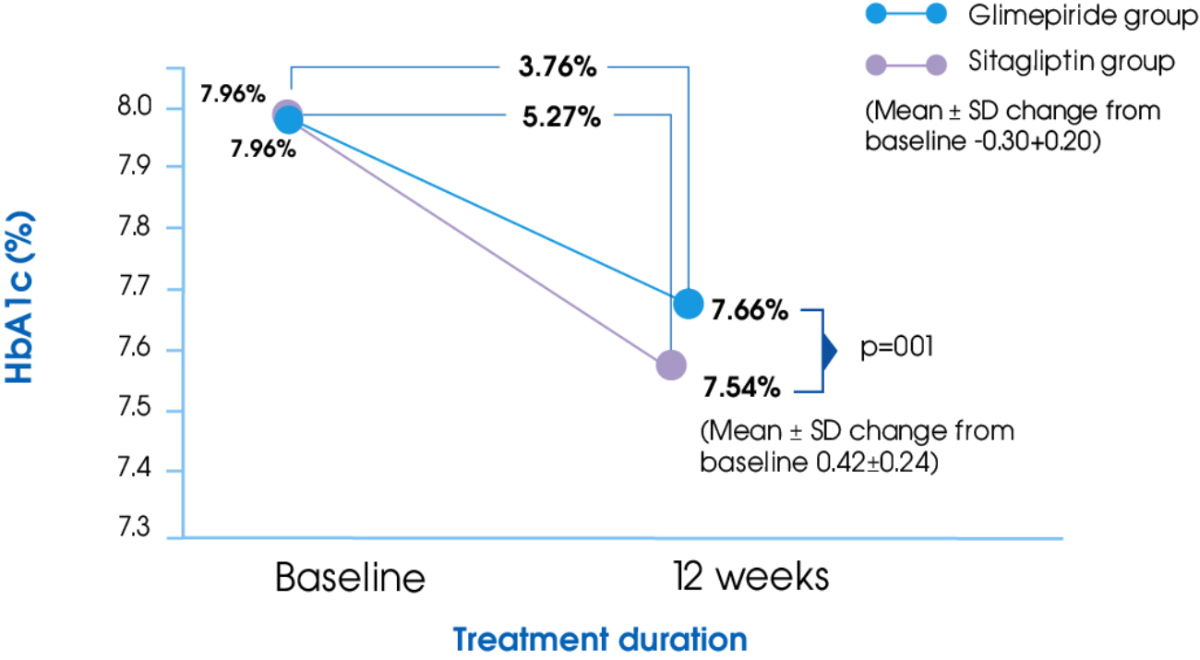
HbA1c2
Glime+Met offers significantly greater reduction in HbA1c compared to Sita+Met2
- Kumar S, Pathak AK, Saikia D, et al. Efficacy, Safety and Treatment Satisfaction of Glimepiride vs Sitagliptin in Combination with Metformin in Type 2 Diabetes Mellitus. Journal of Clinical and Diagnostic Research. 2015;9(12): FC07-FC10.
- Devarajan TV, Venkataraman S, Kandaswamy N, et al. Comparative evaluation of safety and efficacy of glimepiride and sitagliptin in combination with metformin in patients with type 2 diabetes mellitus: Indian multicentric randomized trial - START Study. Indian Journal of Endocrinology and Metabolism. 2017; 21(5):745-750.
Amaryl® Additional Benefits
Amaryl® provides effective glycaemic control with weight neutral effects1
Treatment with Amaryl® resulted in significant and stable weight loss relative to baseline in patients at a higher BMI values*
Mean intra-individual changes from baseline in body weight and HbA1c1
Study design:
Open, controlled, observational study. The primary investigation parameters were changes in HbA1c and body weight relative to baseline in 4 months, 1 and 1.5 years. 1770 T2DM patients were enrolled and 284 were followed-up for 1.5 years. Patients received 0.5 to > 4 mg glimepiride once daily, baseline HbA1c: 8.4%; Body weight 79.8 kg
*Although weight gain was observed in patients with a BMI of < 25 kg/m2, body weight decreases were seen at higher BMI, with the greatest reduction observed in the > 30 kg/m2 class.
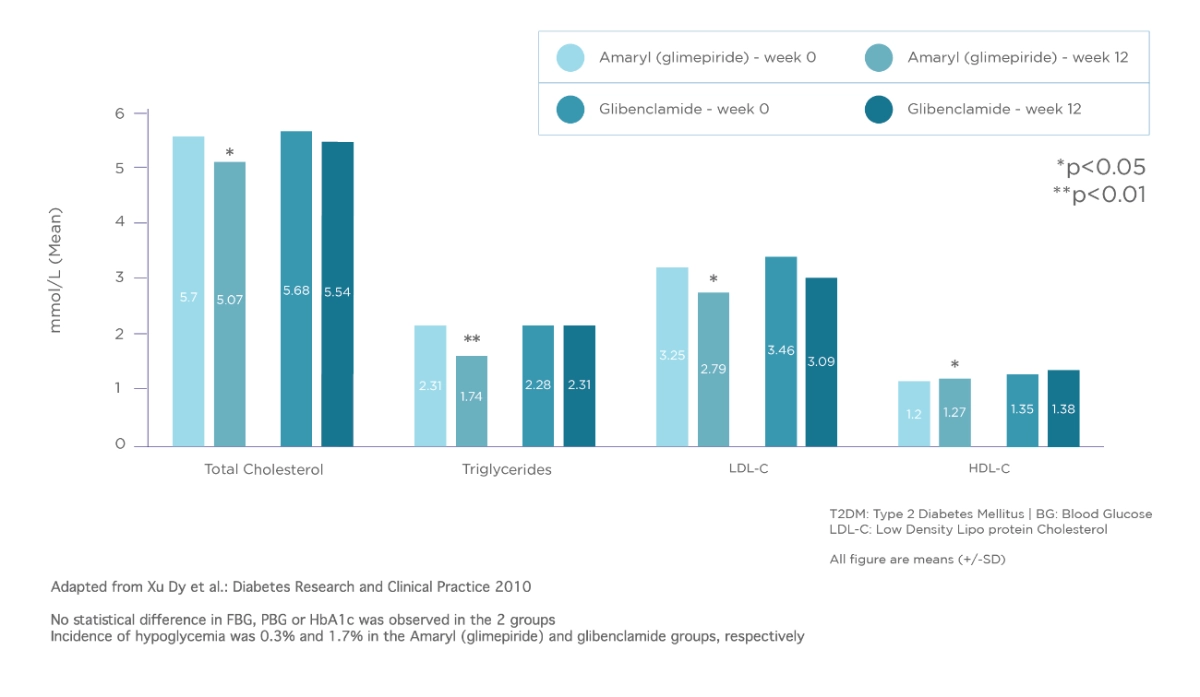
Amaryl® improves lipid profile of newly diagnosed T2DM patients2
Study design:
Randomised study in newly diagnosed T2DM patients investigating the effects of Amaryl® (glimepiride) on BG, plasma lipoproteins and plasminogen activity. Data are from 565 patients randomized to receive Amaryl® 1-2mg once a day (n=333) or glibenclamide 2.5mg once or twice a day (n=232) for 12 weeks.
Objective:
To investigate the effects of Glimepiride on blood glucose in patients with newly diagnosed type 2 diabetes mellitus (T2DM) in connection with plasma lipoproteins and plasminogen activity.
Methodology:
A total of 565 T2DM patients were received Glimepiride (n=333) or Glibenclamide (n=232) for 12 weeks. We observed the level of blood glucose (BG), glycated hemoglobin (HbA1C), the insulin resistance (IR) state, plasma lipoproteins, tissue-type plasminogen activator (t-PA) and plasminogen activator inhibitor type 1 (PAI1-) before and after a 12 weeks of treatment.
- Weitgasser R, et al. Diabetes Res Clin Pract 2003; 61 (1 ): 13-19.
- Dan-yan Xu et al., Diabetes Research and Clinical Practice, 2010.
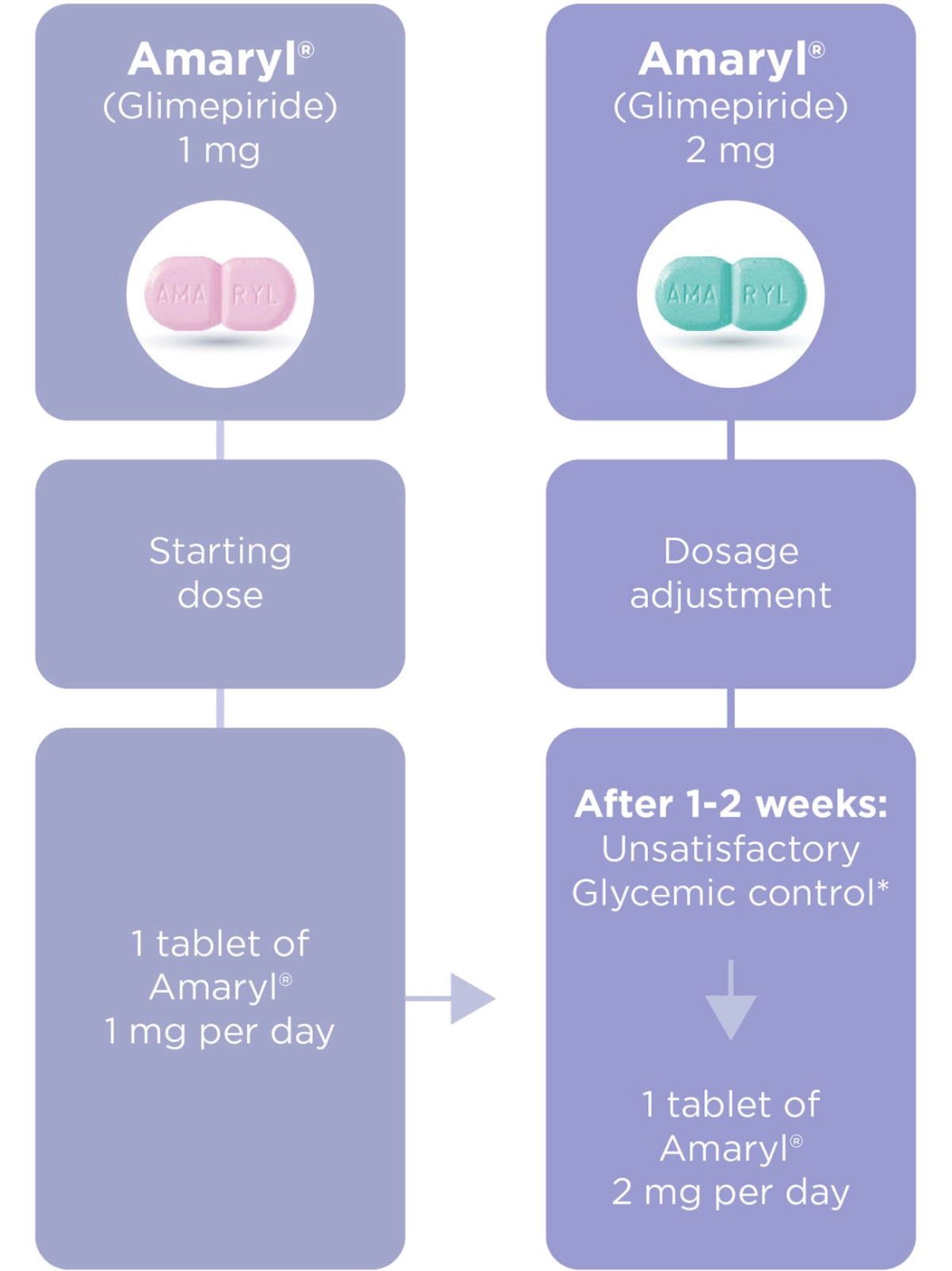
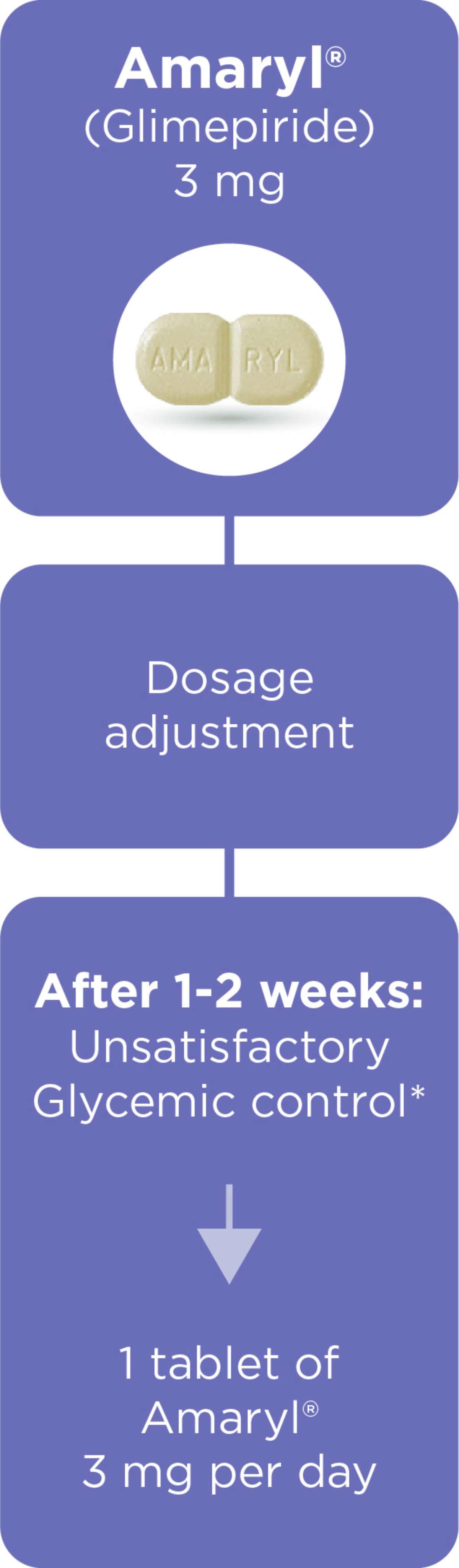
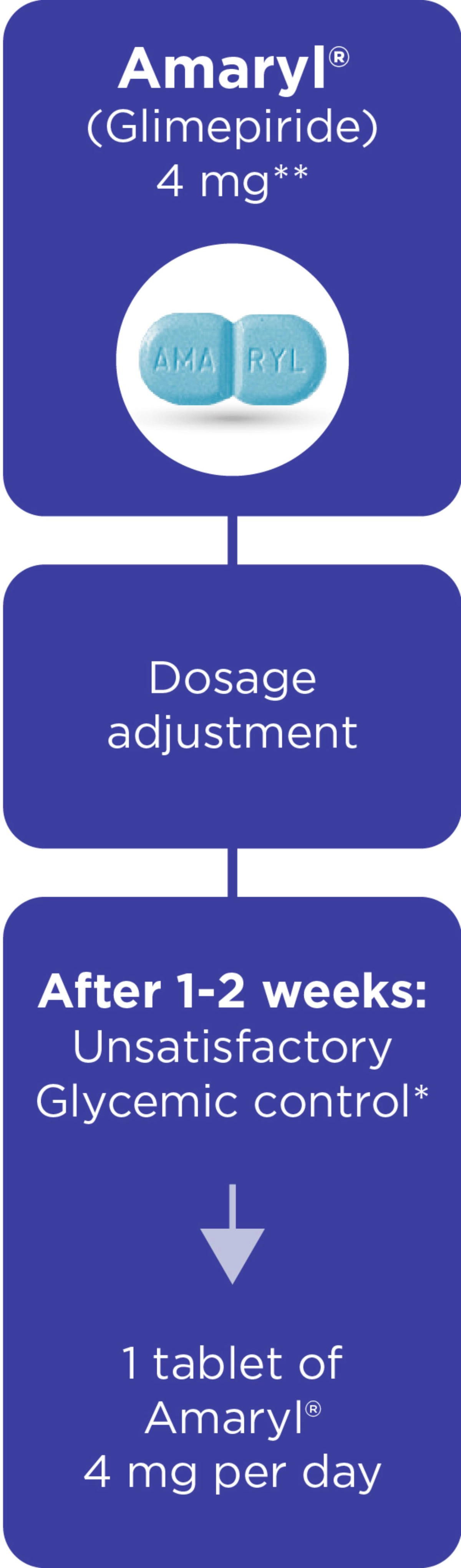
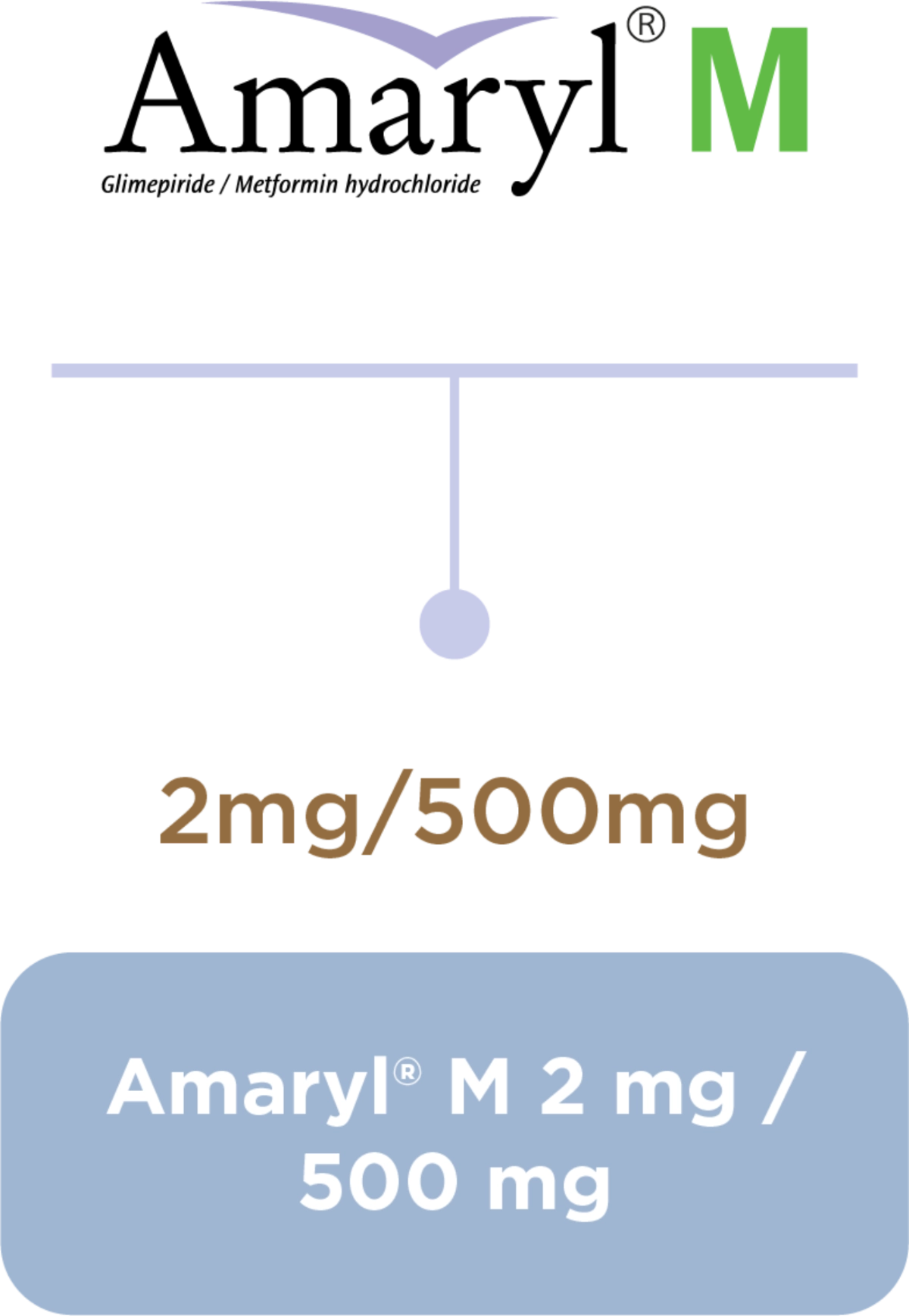
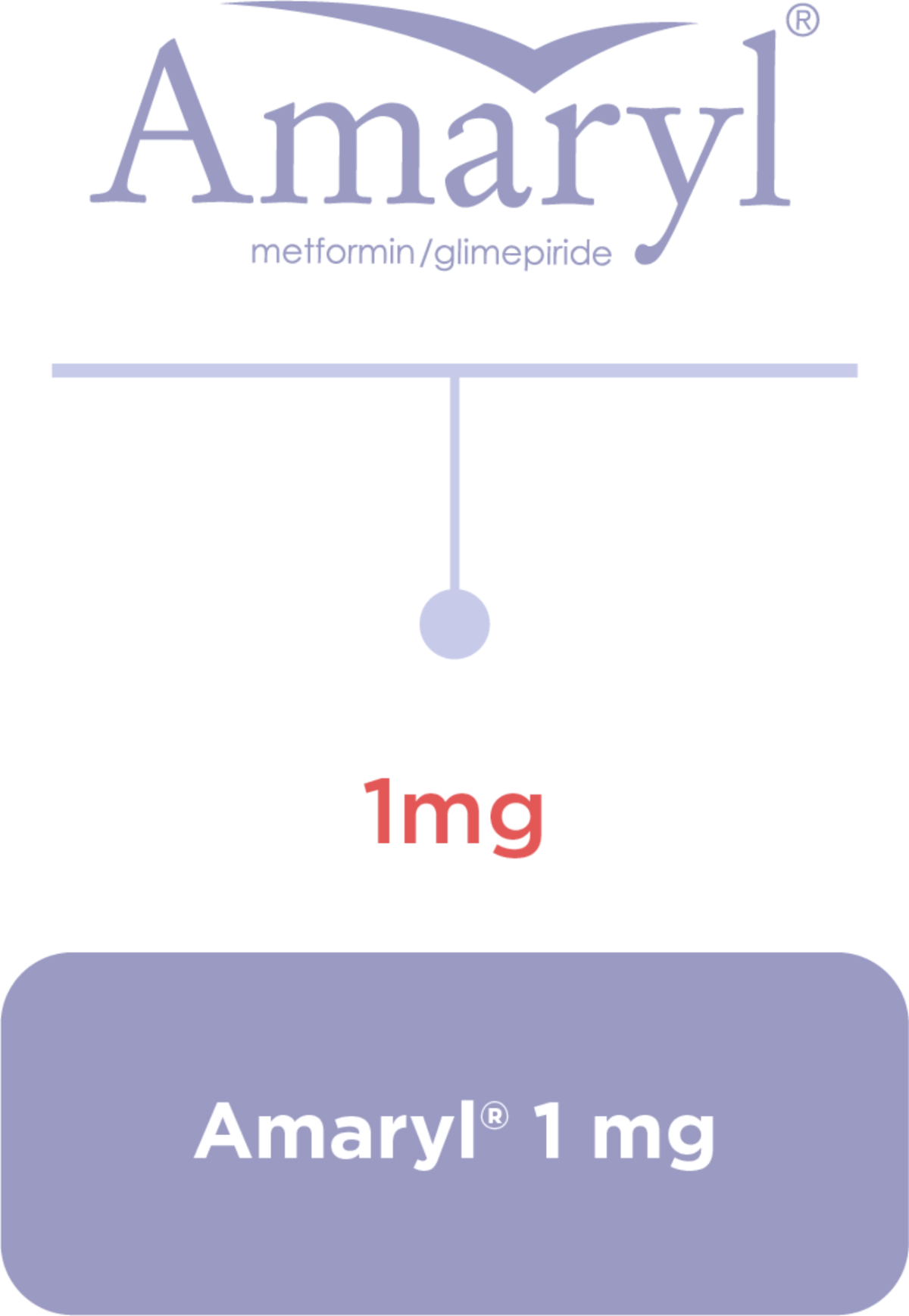
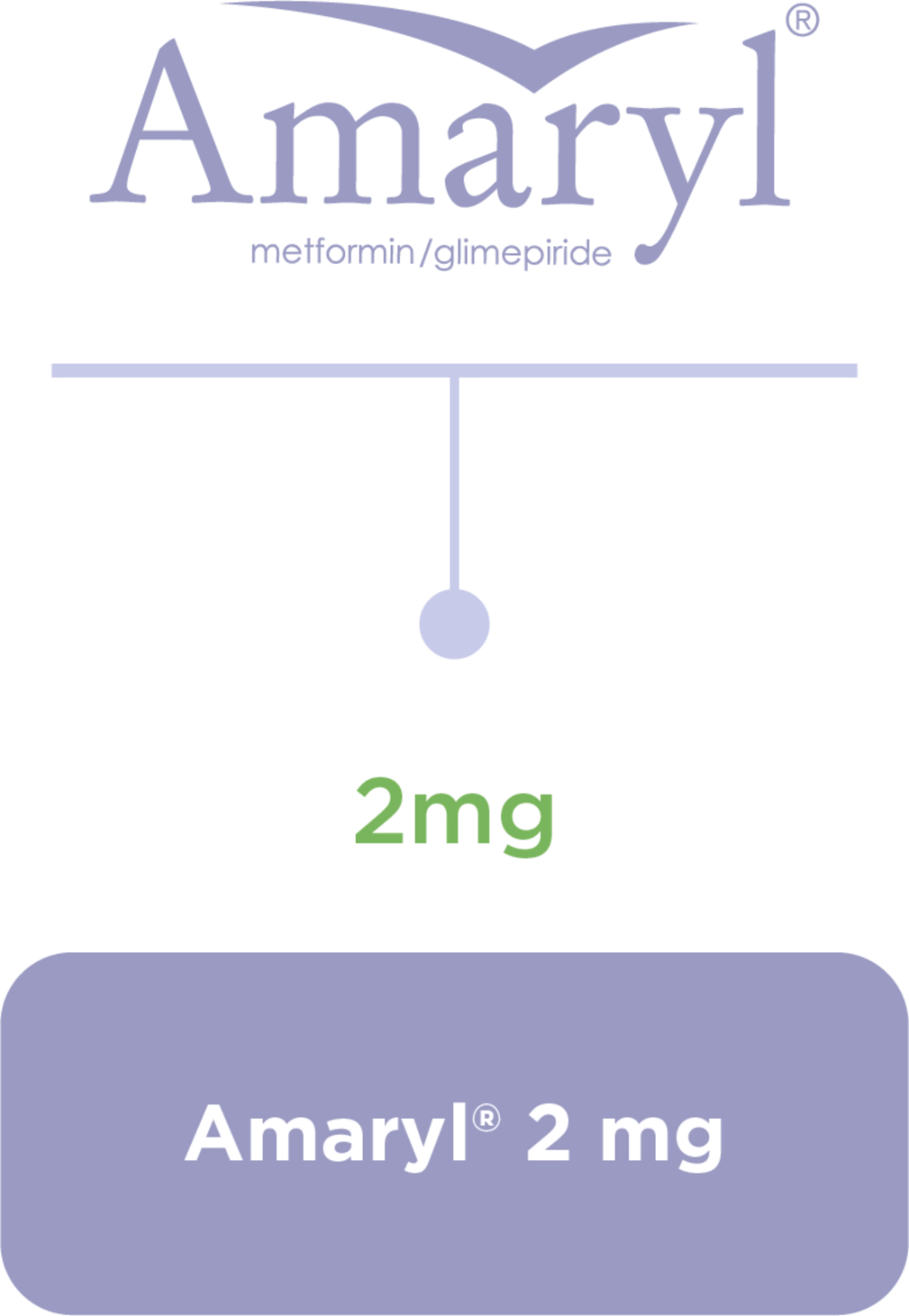
Amaryl® Dosage and Portfolio
Dosage regimen1,3
*The dose of Amaryl depends on your needs, condition and results of blood and urine sugar tests and is determined by your doctor.
Equip your journey with the right balance1,2
- Amaryl® SMPC used in Gulf Countries - last revised in April 2017.
- Amaryl® M SMPC used in Gulf Countries - last revised in December 2016.
- Amaryl® package insert leaflet used in Gulf Countries - last revised in April 2017.
Diabetes is a chronic disease that affects many. Your role as a pharmacist is crucial in helping seek the right help and treatment.
Diabetes Mellitus is a chronic disease.
What are the symptoms?
- Fasting blood sugar level≥126 mg/dL, or
- Random blood sugar level ≥200 mg/dL. or
- HbA1c ≥6.5%
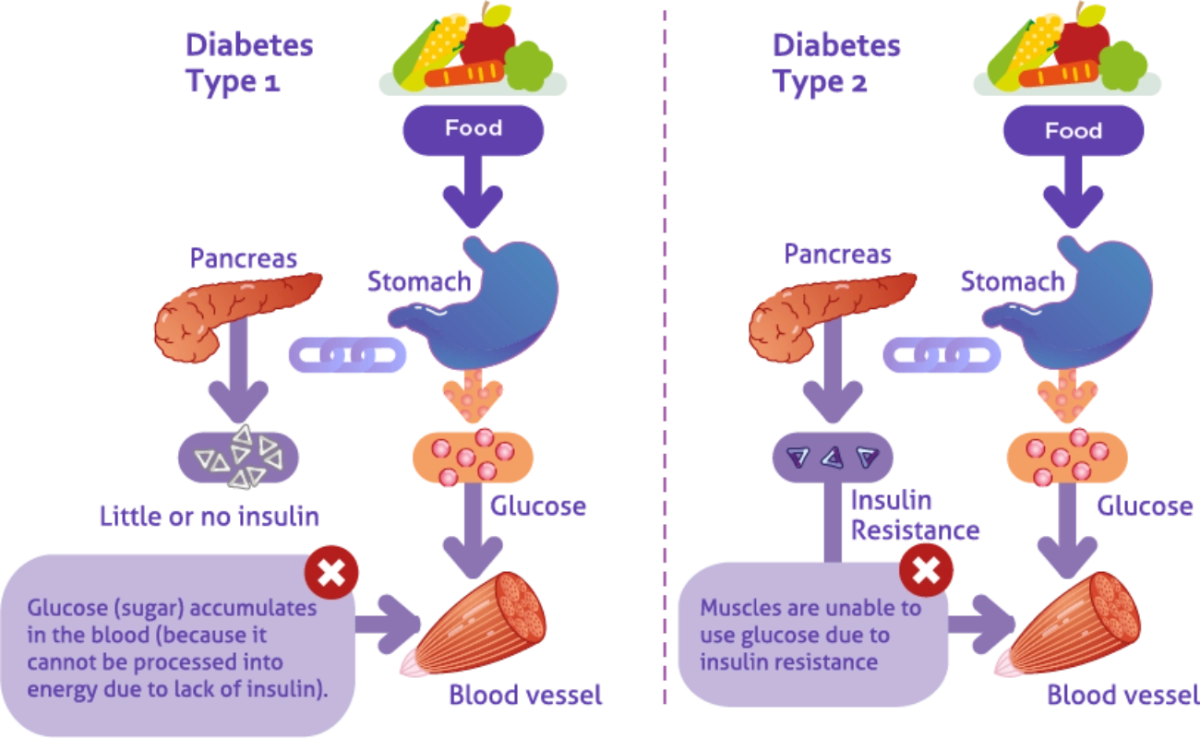
Why does this happen?
- The pancreas does not produce enough insulin
- The body cannot effectively use insulin
- Or both
What are the types of Diabetes Mellitus?
- Diabetes Type 1
- Diabetes Type 2
- Gestational diabetes
- Other types of Diabetes caused by genetic disorders
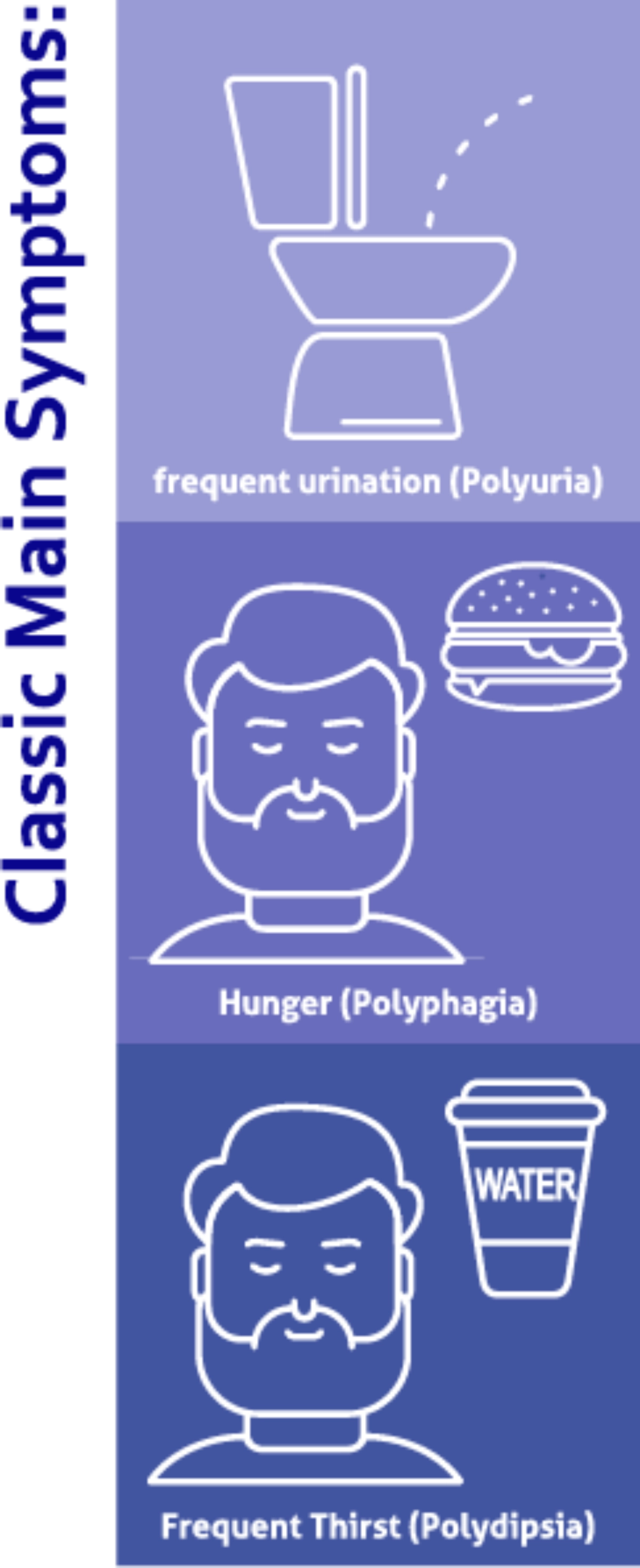
Symptoms of Diabetes Mellitus2
Risk Factors3
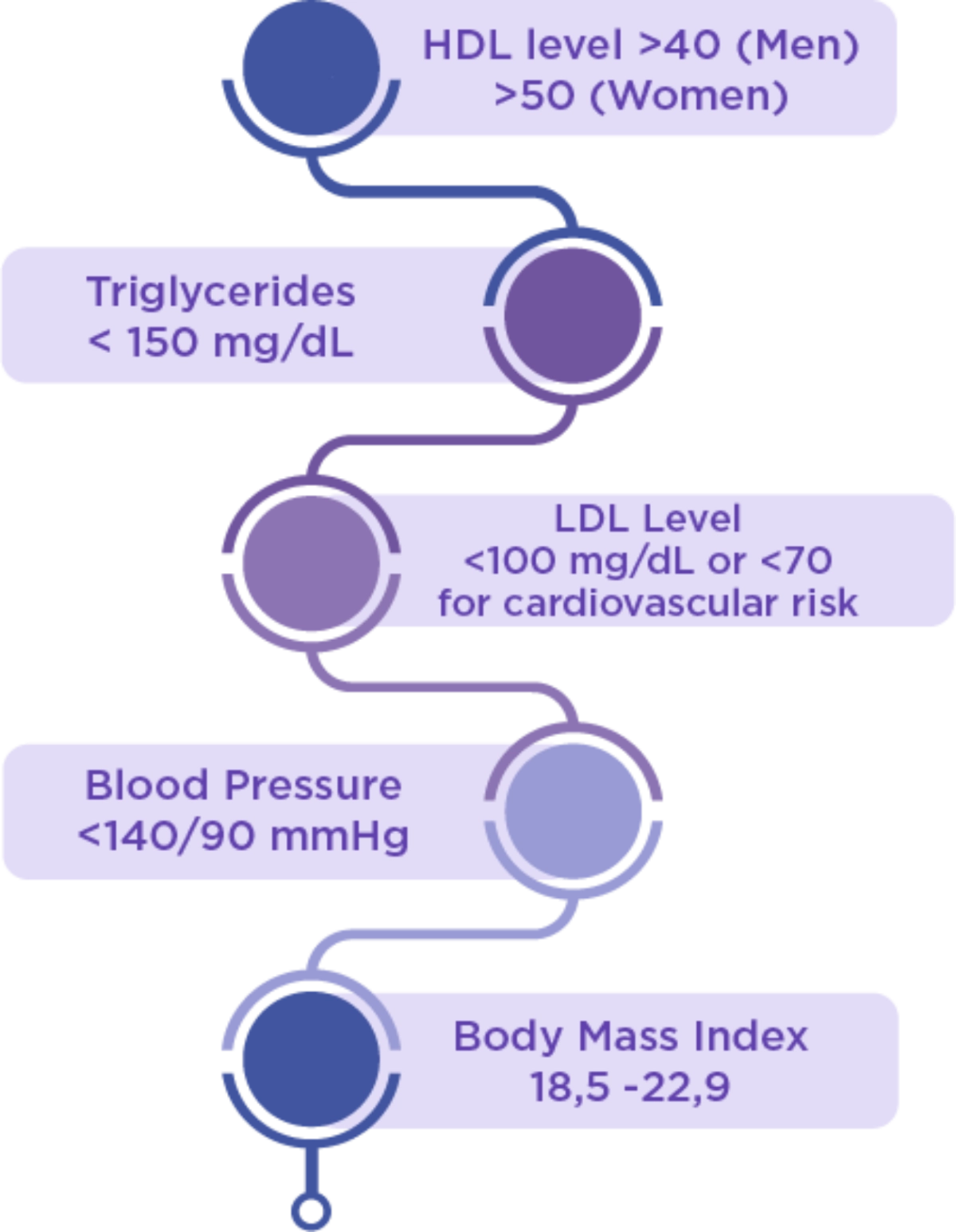
- Blood pressure ≥ 140/90 mm/Hg
- High blood cholesterol levels, HDL <35 mg/dL, and/or triglycerides levels >250 mg/dL
- Record of impaired glucose tolerance(IGT) or impaired fasting glycemia(IFG).
- HbA1c ≥ 5.7%
- Obesity (BMI) ≥ 25kg/m2
- Age (>45 year old)
- Family history
- History of giving birth to baby weighing > 4 kg
- Low birth weight (<2.5kg)
- History of heart disease
- Center for Disease Control. What is Diabetes? CDC. Accessed from website: https://www.cdc.gov/diabetes/basics/diabetes.html [Last reviewed July 7 2022] [Last accessed August 18 2022].
- Center for Disease control. Symptoms of Diabetes. CDC. Accessed on website: https://www.cdc.gov/diabetes/basics/symptoms.html. [Last reviewed April 27 2021] [Last accessed 18 August 2022]
- PB perkini. 2019. Guidelines for the Management and Prevention of Type 2 Diabetes Mellitus in Indonesia. P.64
Diabetes is a chronic disease that affects many. Your role as a pharmacist is crucial in helping seek the right help and treatment.
Amaryl® is a sulfonylurea indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.3 Learn more about Amaryl and the types of patients who can benefit from it.
The right patient profiles for Amaryl®3,4
1. Patients with type II diabetes mellitus with uncontrolled hyperglycemia and need additional use of metformin along side dieting and exercising.
2. Patients with hyperglyceia that cannot be controlled by diet and exercise.
Amaryl® is indicated for use together with insulin.
How does Amaryl® work in the body?
- Amaryl® is a secretory stimulant with dual activity, namely stimulation of insulin action in peripheral tissues.3
- It acts one hour after administration and is completely absorbed with plasma protein binding, up to 99.4%.4
- Decrease in maximal glucose and insulin levels occurs within 2-3 hours of administration,lasting up to 24 hours.4
- Intake of Amaryl® 1 mg once a day for 14 consecutive weeks can reduce: Fasting Blood Glucose: 43mg/dL HbA1c:1.2% Random Blood Glucose: 63 mg/dL3 3
What are Amaryl® co-indications?3,4
- Rosak C. The pathophysiologic basis of ecacy and clinical experience with the new oral antidiabetic agents. Journal of Diabetes and its complications. 16(2012)0231-32.
- Sonnenberg GE, et al. Ann Pharmacother 1997; 31 (6): 671
- Amaryl® package insert leaflet used in Gulf Countries - last revised in June 2015.
- Amaryl®M package insert leaflet used in Gulf Countries - last revised in June 2015.
Diabetes is a chronic disease that affects many. Your role as a pharmacist is crucial in helping seek the right help and treatment.
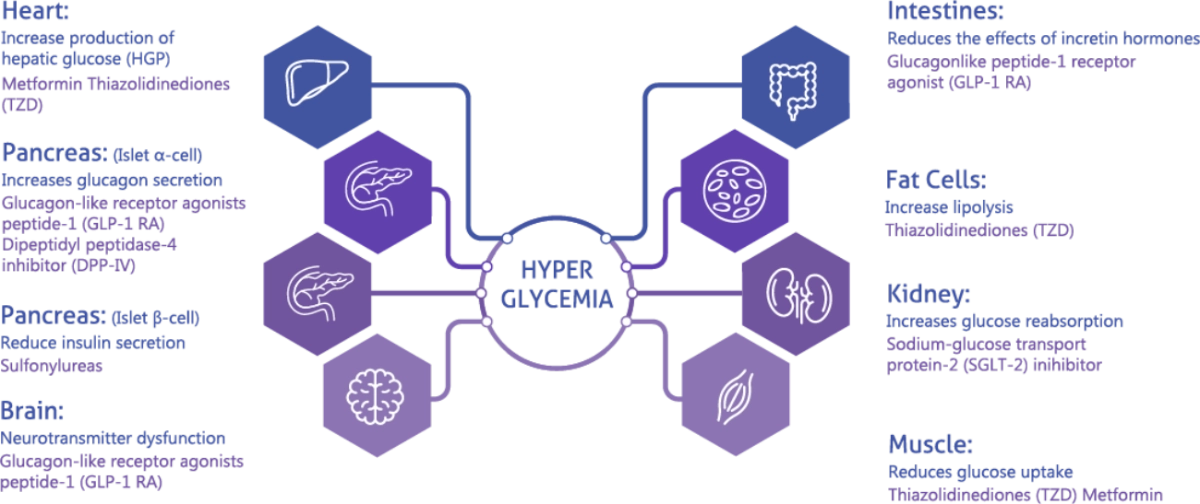
Understanding how antidiabetic medicine work in preventing hyperglyciemia can improve treatment choices. Join us in learning more about the mechanism of action of antidiabetic medication and the efficacy of sulfonylureas as mono and combination therapy.
Sulphonylurea Efficacy
| Monotherapy | |
| Therapy Regimen | HbA1c decrease4 (%) |
| Sulphonylurea (mono) | 1.0-2 |
| Glimide | 0.5-1.5 |
| Metformin | 1.0-1.4 |
| Alfa-Glucosidase Inhibitor | 0.5-0.8 |
| Tiazolidinedion | 0.5-1.4 |
| DPP-4 inhibitor | 0.5-0.8 |
| SGLT-2 inhibitor | 0.8-1.0 |
| Combination therapy | |
| Therapy Regimen | HbA1c decrease5 (%) |
| Sulphonylurea + metformin | 1.7(16) |
| Sulphonylurea + rosiglitazone | 1.4(18) |
| Sulphonylurea + pioglitazone | 1.2(19) |
| Sulphonylurea + acarbose | 1.3(20) |
| Repaglinide + metformin | 1.4(17) |
| Poiglitazone + metformin | 0.7(21) |
| Rosiglitazone + metformin | 0.8(22) |
| Dipeptidyl peptidase 4 inhibitor + metformin | 0.7(23) |
| Dipeptidyl peptidase 4 inhibitor + pioglitazone | 0.7(23) |
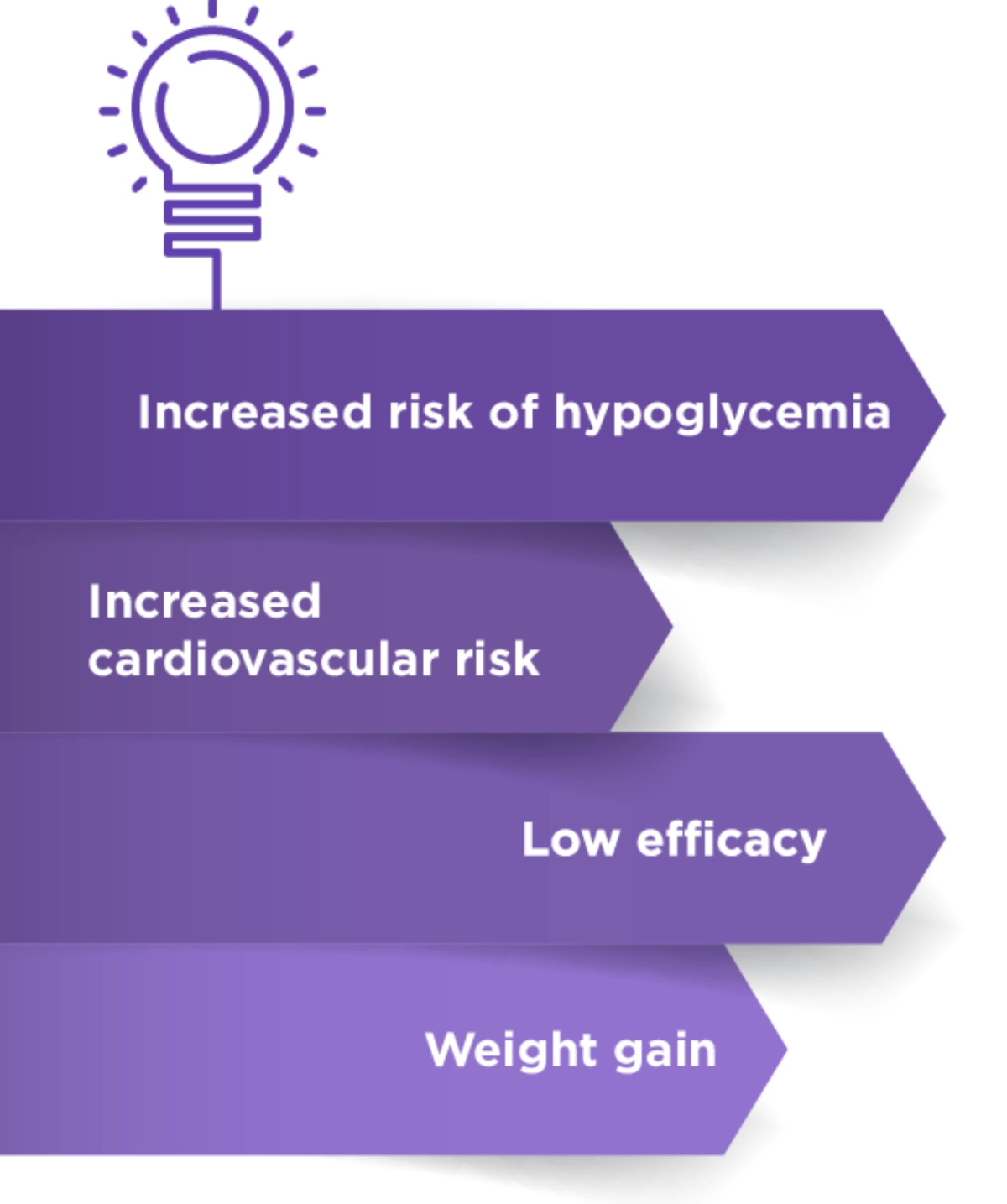
Glimepiride Fact Check6
Concerns6
Facts
Glimepiride is associated with a lower hypoglycemic effect due to:
- Stimulation of insulin secretion
- Lower binding affinity
- Rapid association and dissociation with sulphonylurea receptor
Glimepiride has a cardiovascular safe profile based on a recent CAROLINA study
Glimepiride is clinically proven to reduve hyperglycemic conditions in patients with type 2 diabetes mellitus as mono or combination therapy.
Glimepiride has been reported to have a weight loss or neutralizing effect compared with conventional sulphoneyureas.
- Rosak C. The pathophysiologic basis of ecacy and clinical experience with the new oral antidiabetic agents. Journal of Diabetes and its complications. 16(2012)0231-32.
- Sonnenberg GE, et al. Ann Pharmacother 1997; 31 (6): 671
- Ralph AD. From the trimvitrate to the omnious octet: A New Paradigm for Treatment of Type 2 Diabetes Mellitus. Diabetes. 2009; 58:773-795.
- AACE Diabetes Mellutus Clinical Practive Guidelines Task Force. Endocrine Practice 2007; 13:3-68.
- Fonseca V. Clinical significance of targeting postprandial and fasting hyperglycemia in managing type 2 diabetes mellitus. Current medical research and opinion. 2003; 19(7): 635-641.
- Shunmugavelu M, et al. Myths and facts about Glimepride. JAPI. 2019; 24-26.
Diabetes is a chronic disease that affects many. Your role as a pharmacist is crucial in helping seek the right help and treatment.
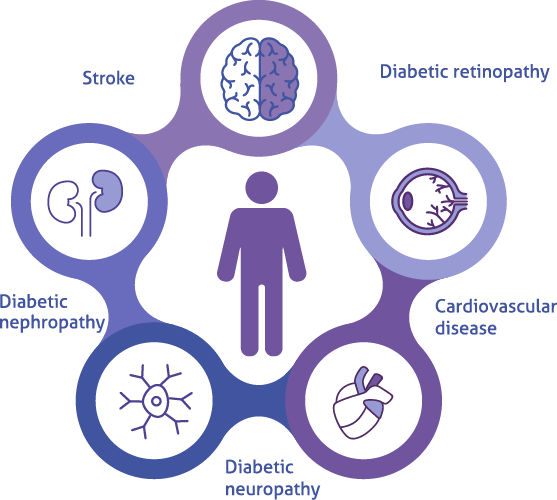
Join us in learning more about the complications of uncontrolled diabetes, the glycemic targets and the different approaches for controlling blood glucose levels.
Complications of Diabetes Mellitus3
Diabetes can result in complications if left uncontrolled.
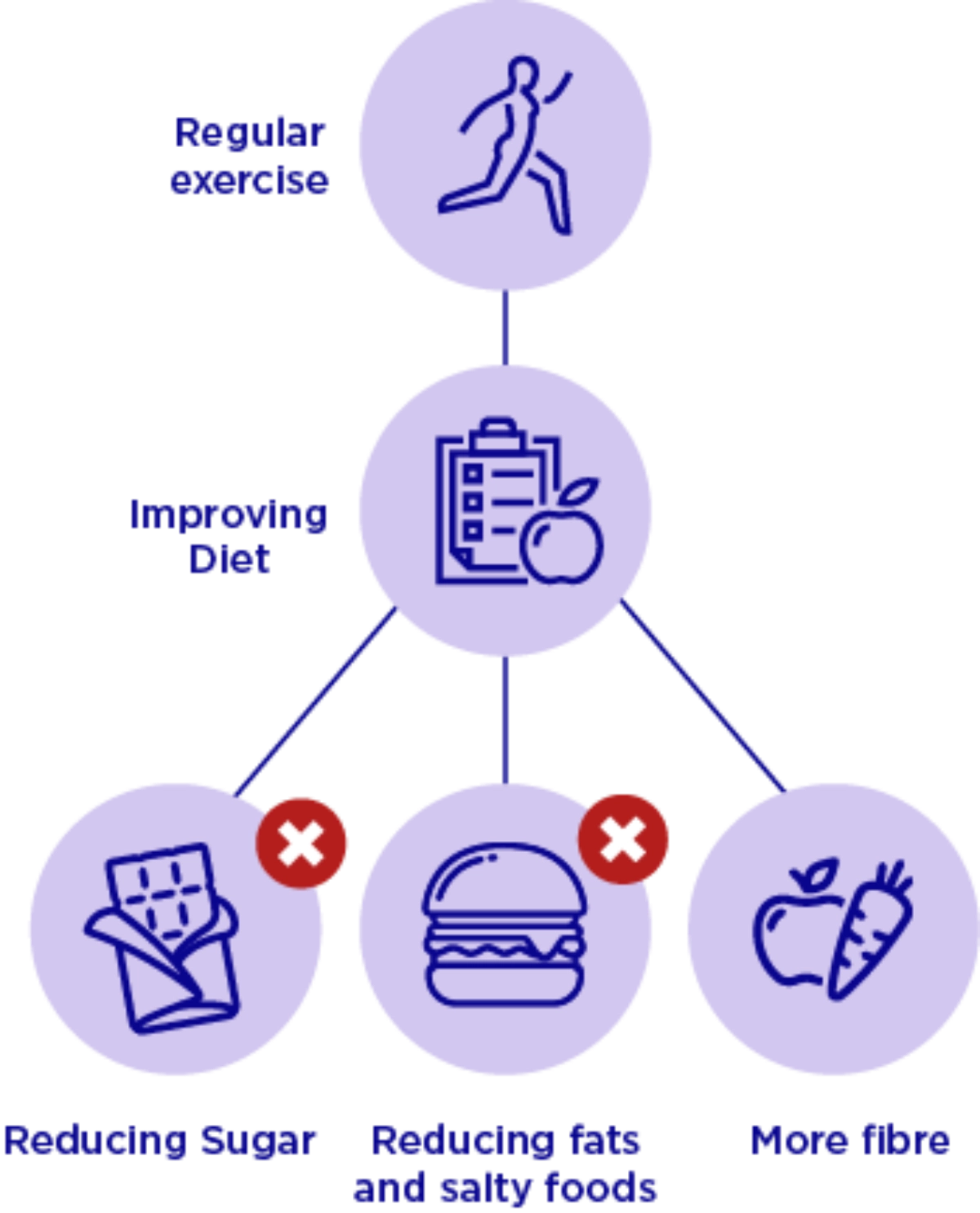
These complications can be prevented3
Lifestyle changes
Monitoring for specific symptoms
- Infections and skin disorders
- Check vision
- Tingling, burning or loss of sensations
- Sores bottom of foot
Pharmacotherapy
- Take medication regularly
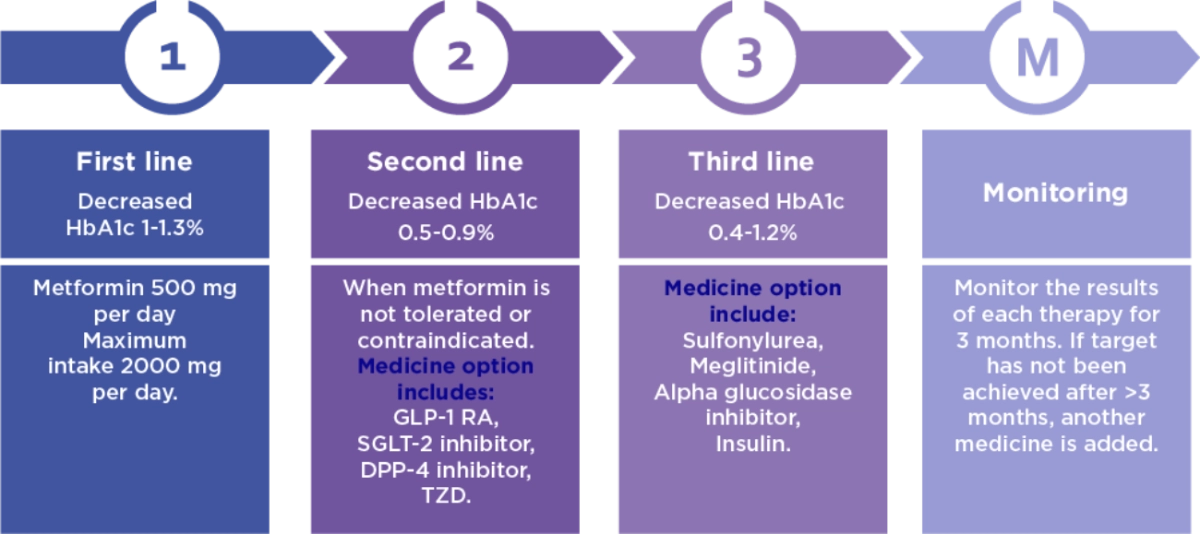
There are several therapy options for diabetes mellitus...
Glycemic Targets |
|
| HbA1c | <7% |
| Fasting blood sugar | 80-110 mg/dL |
| Blood sugar 2 hrs after eating | <180 mg/dL |
Lifestyle Targets
Pharmacotherapy Medicine Selection Strategy
- Rosak C. The pathophysiologic basis of ecacy and clinical experience with the new oral antidiabetic agents. Journal of Diabetes and its complications. 16(2012)0231-32.
- Sonnenberg GE, et al. Ann Pharmacother 1997; 31 (6): 671
- PB Perkini. 2019. Guidelines for the management and prevention of type 2 diabetes mellitus in Indonesia.
MAT-BH-2200260/V3/March 2022

.png)

.jpg/jcr:content/image%20(1).jpg)
.jpg/jcr:content/image%20(2).jpg)
.jpg/jcr:content/image%20(3).jpg)









.webp)