About Tavanic®
Levofloxacin (Tavanic®) is a fluoroquinolone antibacterial agent with broad spectrum of activity against Gram-positive and Gram-negative bacteria and atypical respiratory pathogens. It is effective in the wide range of clinical infections including respiratory tract, urinary tract and skin and skin structure infections (Drugs 2003).
How does Tavanic® work?
Like other fluoroquinolones, Tavanic® inhibits the both bacterial DNA gyrase and topoisomerase IV; the primary enzyme target varies for different species of bacteria.1

What distinguishes Tavanic® from other fluoroquinolones?
Tavanic® exhibits broad-spectrum bactericidal activity, excellent oral bioavailability, good tissue penetration and favorable safety and tolerability profiles.
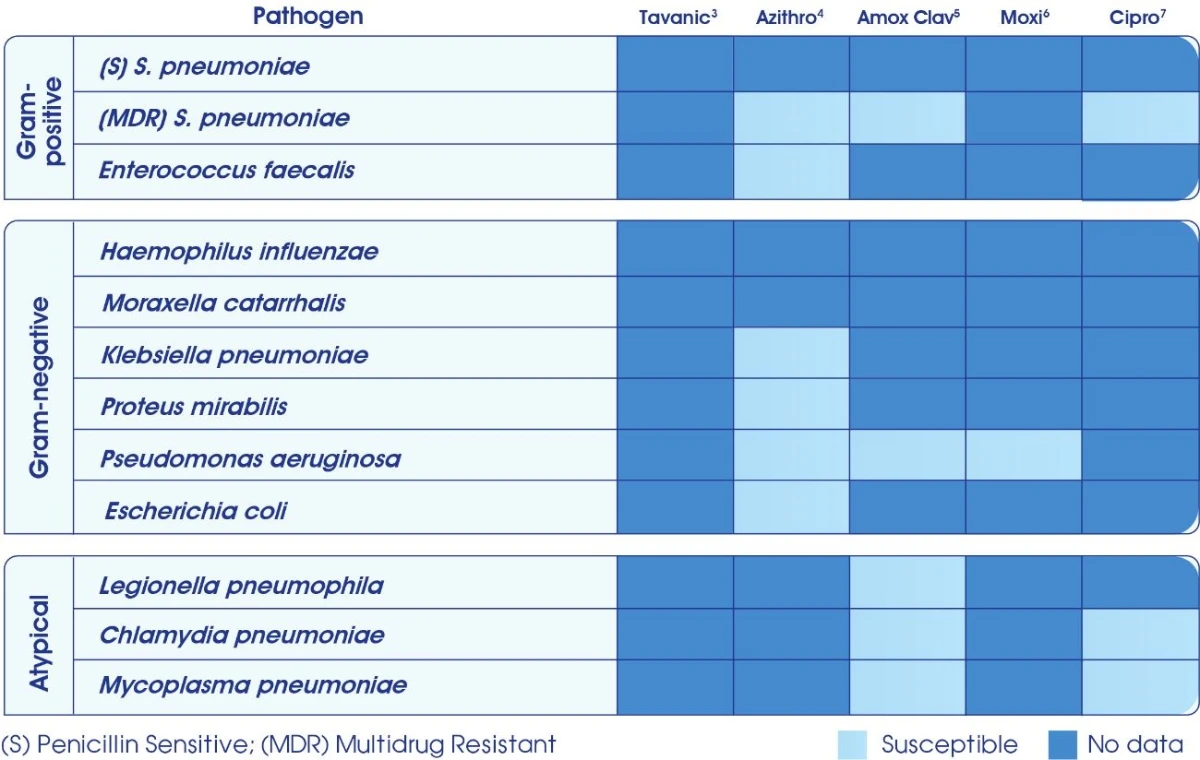
Broad spectrum of activity
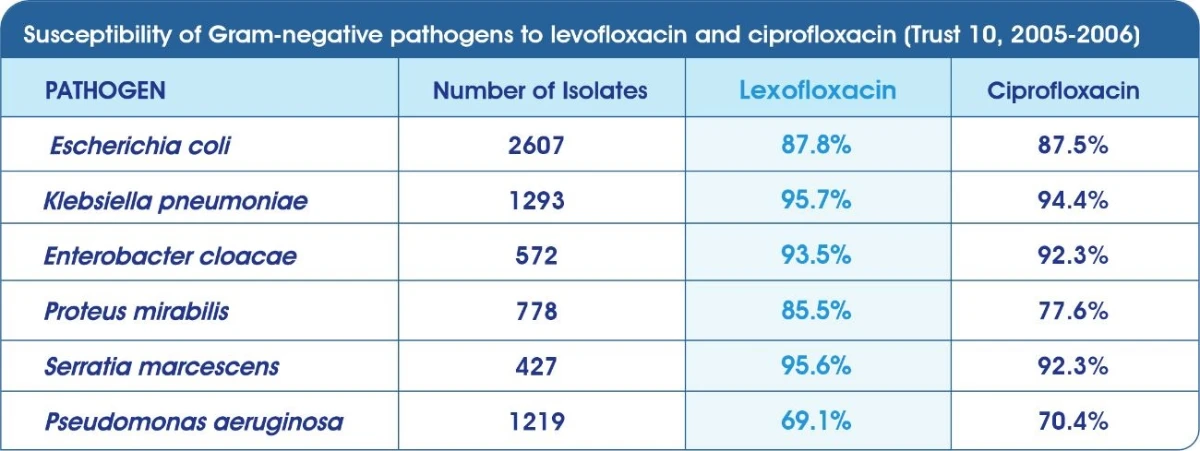
Levofloxacin is currently the only established with broad spectrum activity against susceptible & difficult to treat pathogens.2 Levofloxacin is often bactericidal at concentrations equal to or slightly greater than inhibitory concentrations.1
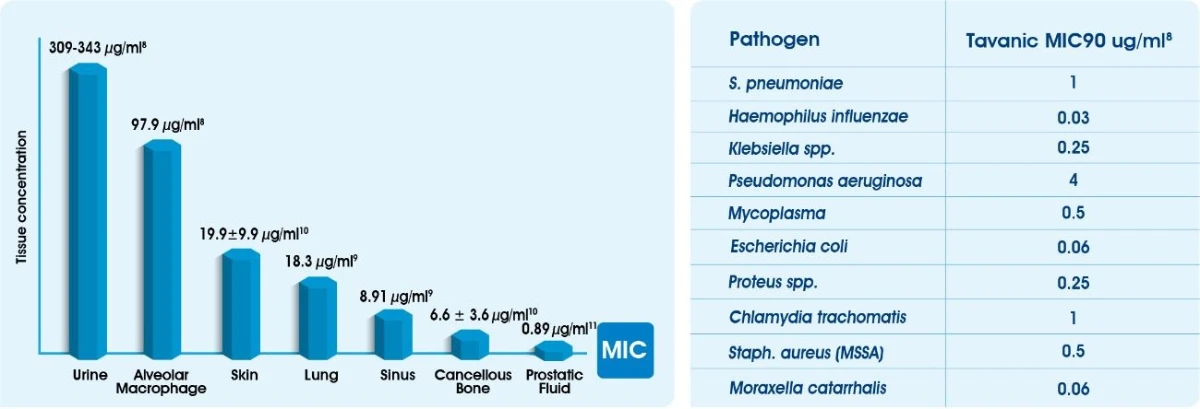
Good Tissue Penetration8-12
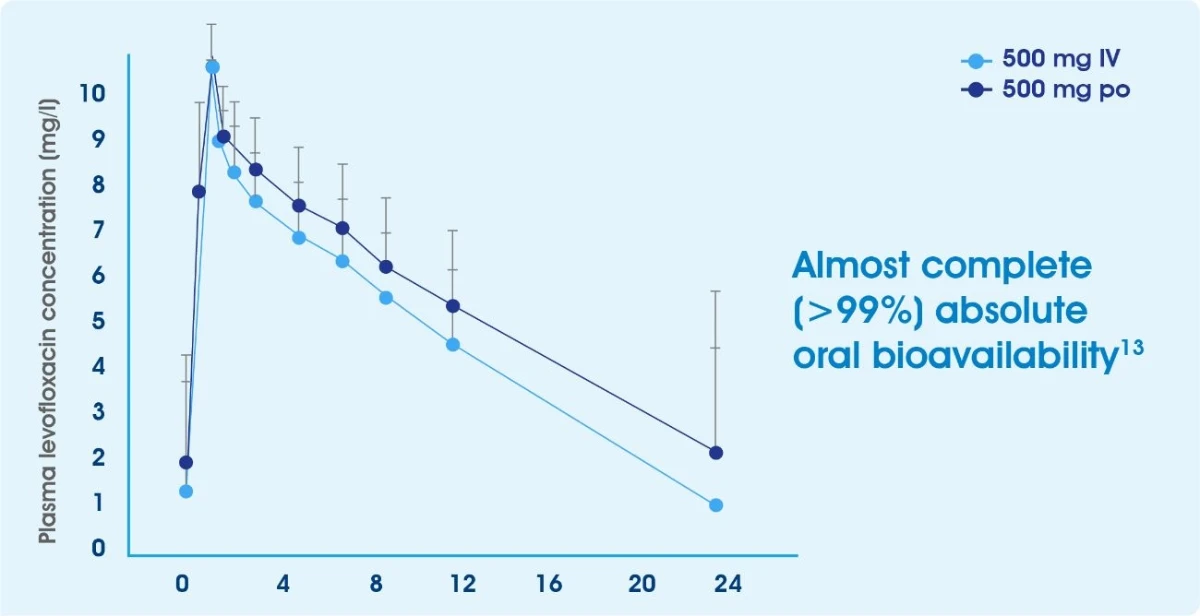
Excellent bioavailability (>99% absorption)13
The absolute bioavailability of the tablet formulation is approximately 99%. Mean steady state levofloxacin plasma concentration-time profiles following IV and oral administration of 500 mg OD during sequential therapy in LRTI patients (n=17)
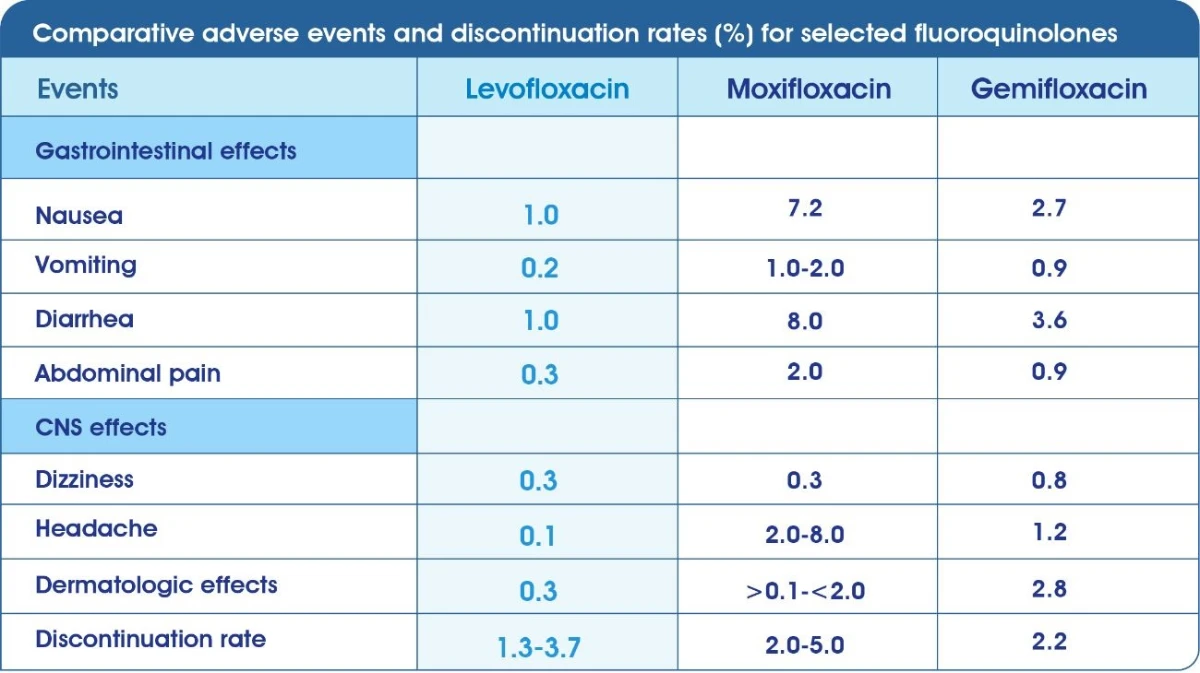
Well-defined Safety & Tolerability:14,15
Tavanic® is also associated with a mild effect on normal microflora, reaching a maximum at 4 days of therapy and complete recovery being achieved by 7 days post-therapy
- Levofloxacin, FDA approval. September 2008
- International Journal of Antimicrobial Agents 28S (2006) S115-S127.
- LEVAQUIN® Prescribing Information. September 2008
- ZITHROMAX® Prescribing Information. January 2013
- AUGMENTIN® Prescribing Information.
- AVELOX® Prescribing Information. October 2008
- CIPRO®Prescribing Information. October 2008
- Tavanic Product monograph. June 2003.
- Clin Microbiol Infect 2005; 11 (Suppl. 2): 653, Abs:R 1965.
- H.V. Baum, UM 18 (2001); 335-340.
- J chemotherapy, Vol 16-supllement n.2 (18-21 )2004 K.G.Naber.
- Kohlhoff SA, Roblin PM, Reznik T, Hawser S, Islam K, Hammerschlag MR. In vitro activity of a novel diaminopyrimidine compound, iclaprim, against Chlamydia trachomatis and C. pneumoniae. Antimicrob Agents Chemother. 2004 May; 48 (5): 1885-6.
- Furia nut M, Brollo L, et al. Pharmacokinetic aspects of Levofloxacin 500 mg once daily during sequential intravenous/oral therapy in patients with lower respiratory tract infections. J Antimicrob chemother 2003; 51(1): 101-6.
- Clinical infectious disease 1999; 28: 352-64. 15. Using Safety Profiles to Differentiate between the Newer Fluoroquinolones Keith A. Rodvold. 2007: 23-30.
Burden of Antibiotic resistance - Tavanic® tackles difficult-to-treat
resistant pathogens
There is global concern about the growing problem of antimicrobial resistance1. Systematic misuse and overuse of antibiotics in human medicine and food production have put every nation at risk leading to treatment failure.1,2 Heavy international travel, large population of expatriate workers, booming tourism are few factors in addition to overuse or misuse of antibiotics in the GCC regions.3
Infections with resistant organisms are difficult to treat requiring costly and sometimes toxic alternatives. While rates of resistance to other previously useful antimicrobial classes has grown, Levofloxacin (Tavanic®) has maintained its efficacy with generally very low rates of resistance around the world.4-8
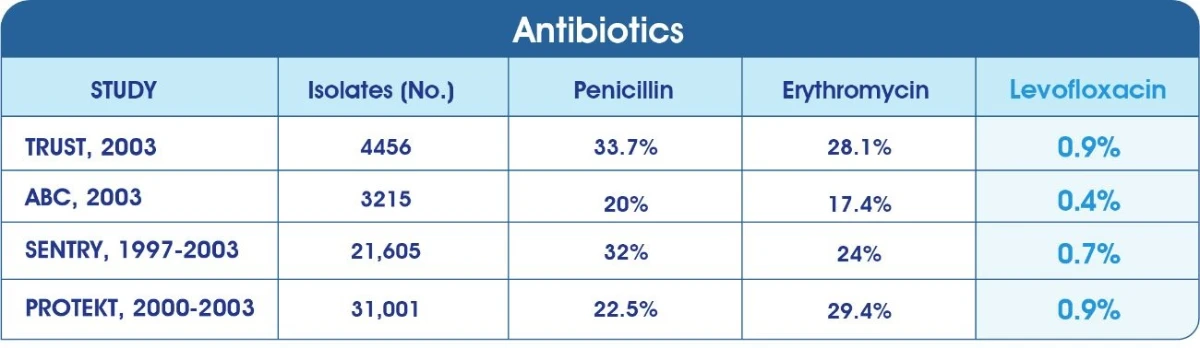
Non-susceptibility rates of Streptococcus pneumonia2-5
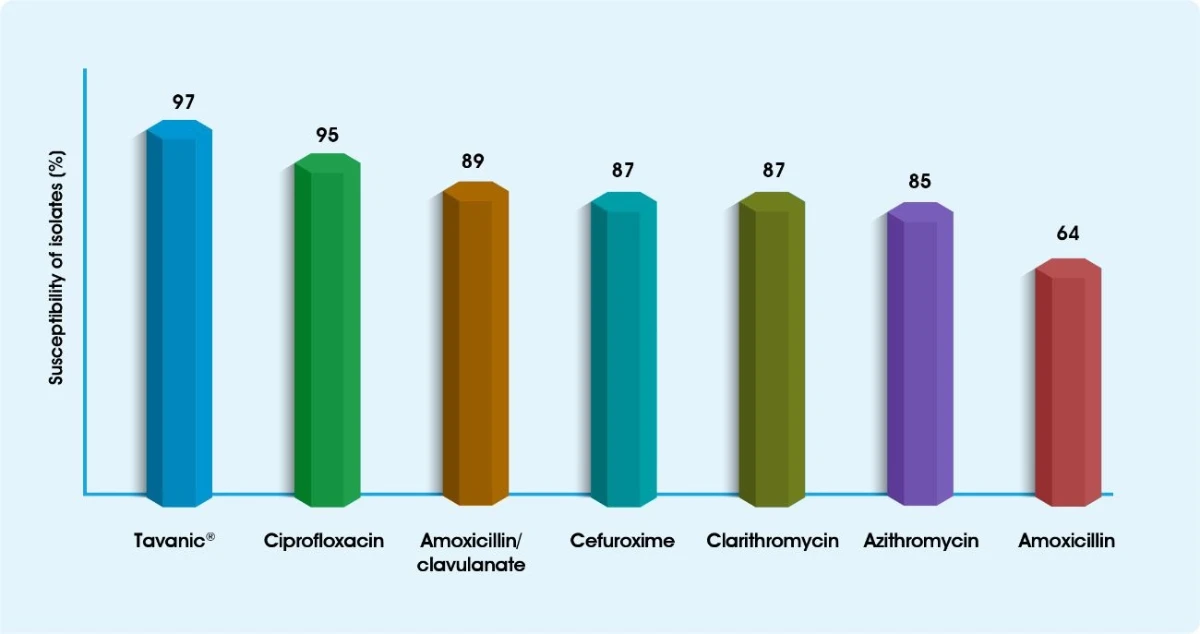
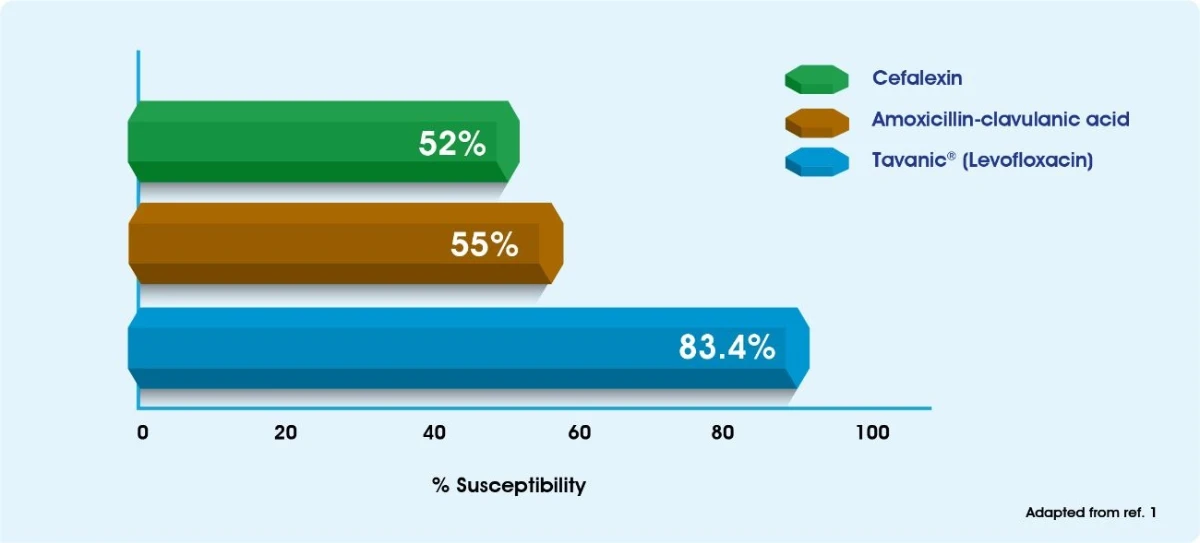
Sensitivity of clinical isolates of S.pneumoniae from Saudi Arabia9
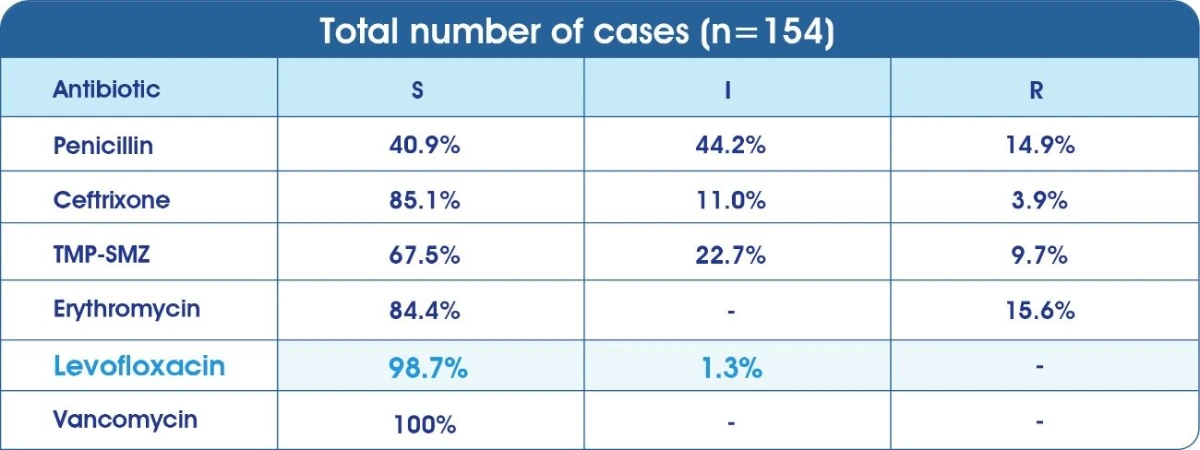
(S) Sensitive (1) Intermediate (R) Resistant
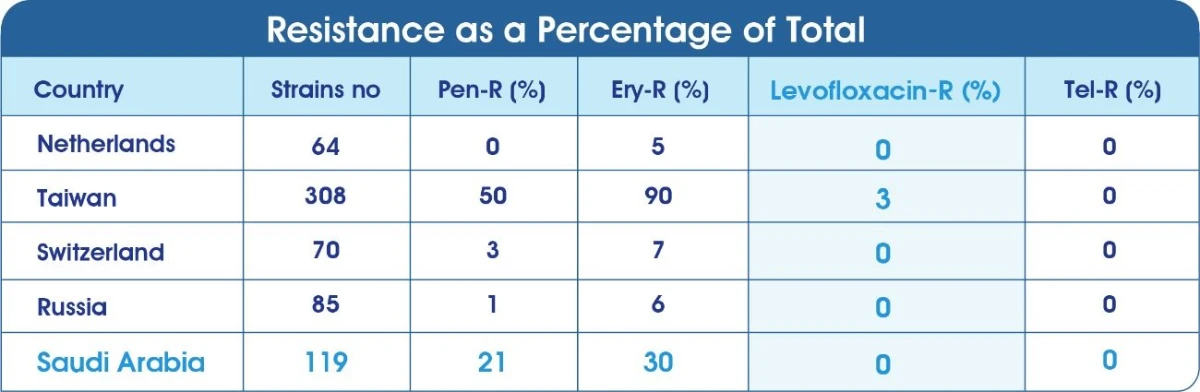
Resistance of S.pneumoniae in Saudi Arabia10
Antibacterial resistance among Streptococcus pneumoniae isolated in Europe, Turkey, Middle East, Africa and Asia (eBASKET2) 2003
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-655. doi:10.1016/S0140-6736(21)02724-0
- Manyi-Loh C, Mamphweli S, Meyer E, Okoh A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules. 2018;23(4):795. Published 2018 Mar 30. doi:10.3390/molecules23040795
- Zowawi HM. Antimicrobial resistance in Saudi Arabia. An urgent call for an immediate action. Saudi Med J. 2016;37(9):935-940. doi:10.15537/smj.2016.9.16139
- CDC. Antibiotic/Antimicrobial Resistance. About Antimicrobial Resistance. 2013 available at: http://www. cdc. gov/drug resistance/about. html. last accessed: 25.8.2015.
- Karlowsky JA,Thornsberry C, Jones ME, et al. Factors associated with relative rates of antimicrobial resistance among Streptococcus pneumoniae in the United States: results from the TRUST Surveillance Program (1998-2002). Clin Infect Dis 2003; 36 (8): 963-70.
- CDC. ABCs Report: Streptococcus pneumoniae, 2003. Available at: http://www.cdc.gov/abcs/ reports-findings/survreports/spneu03.html. Last accessed: 13.9.015.
- Jones RN, Sader HS, Mendes RE, Flamm RK. Update on antimicrobial susceptibility trends among Streptococcus pneumoniae in the United States: report of cettaroline activity from the SENTRY Antimicrobial Surveillance Program (1998-2011 ). Diagn Microbiof Infect Dis 2013; 75(1 ): 107-9.
- Farrell DJ, Jenkins SG, Brown SD, Patel M, Lavin BS, Klugman KP. Emergence and spread of Streptococcus pneumoniae with erm(B) and mef(A) resistance. Emerg Infect Dis 2005; 11 (6):851-8.
- Z.A. Memish, et al. International Journal of Antimicrobial Agents 23 2004; 32-38.
- Medscape: Multispeciality. CME and Education. CommunityAcquired Pneumonia. 2004 Available at: http://www.medscape.org/viewarticle/495508. Last accessed: 24.8.2015.
Efficacy Studies
Tavanic® in the management of respiratory tract infections.
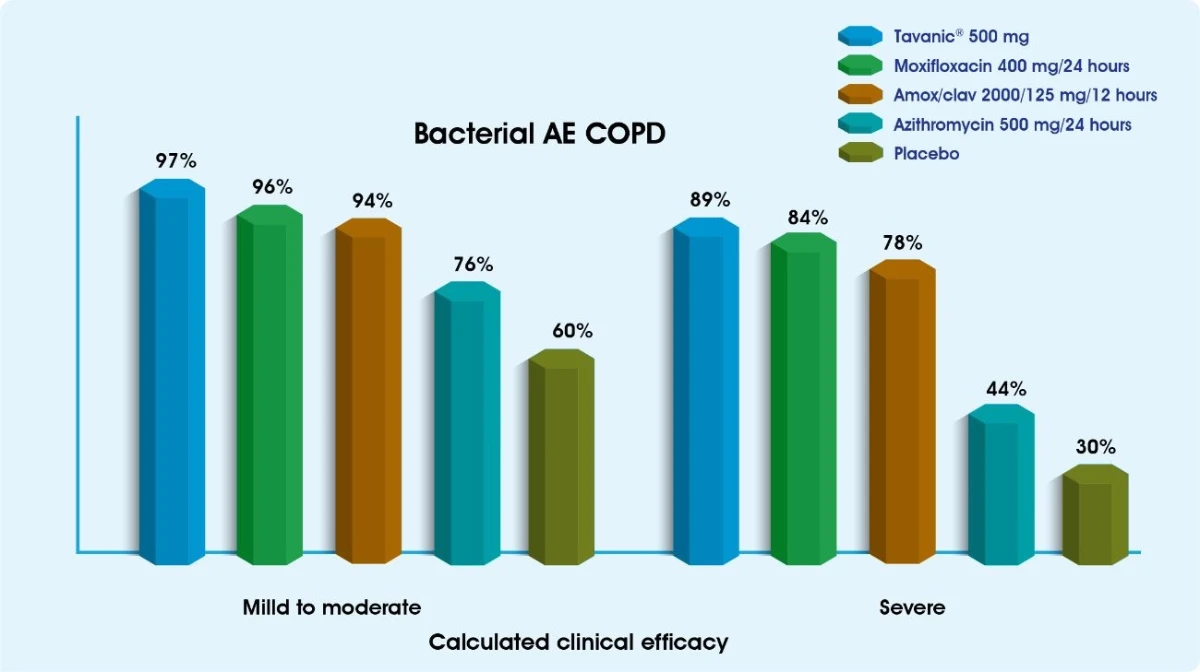
Tavanic® has proven to be highly effective in the management of AECB1,2
The efficacy of Tavanic® for the treatment of pneumonia has been studied extensively3,4
Community acquired pneumonia
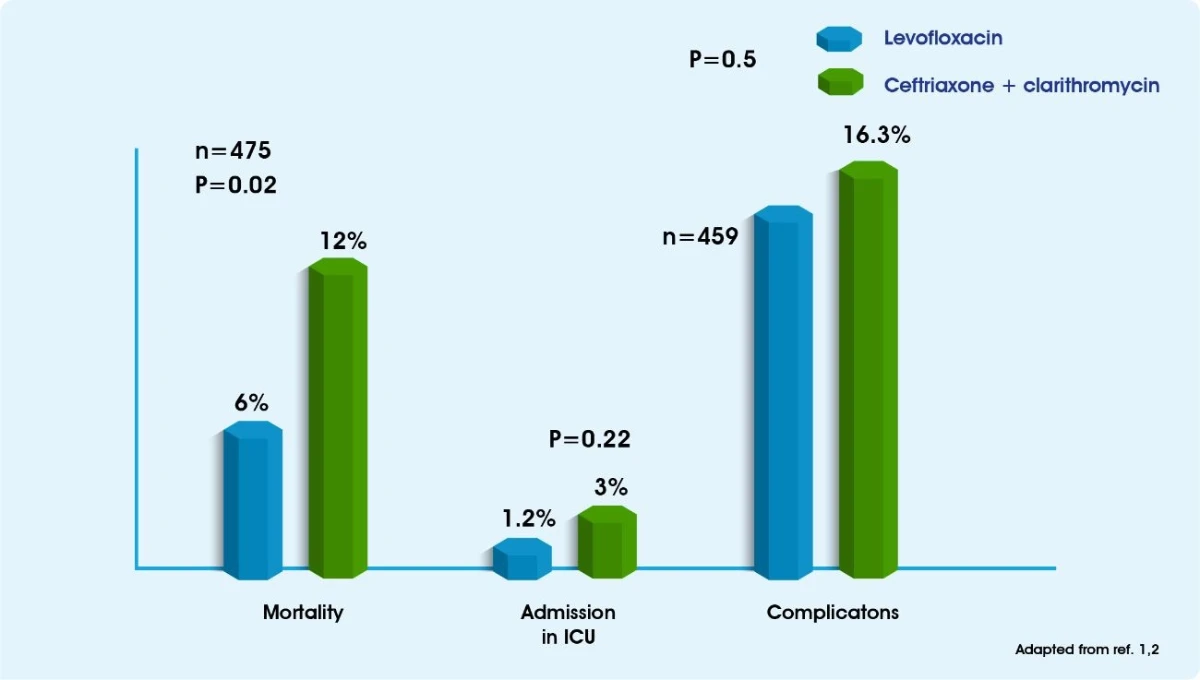
Monotherapy with Levofloxacin has been shown to be as effective as combination therapy with a beta lactam plus a macrolide for the treatment of patients with CAP requiring hospital admission.3
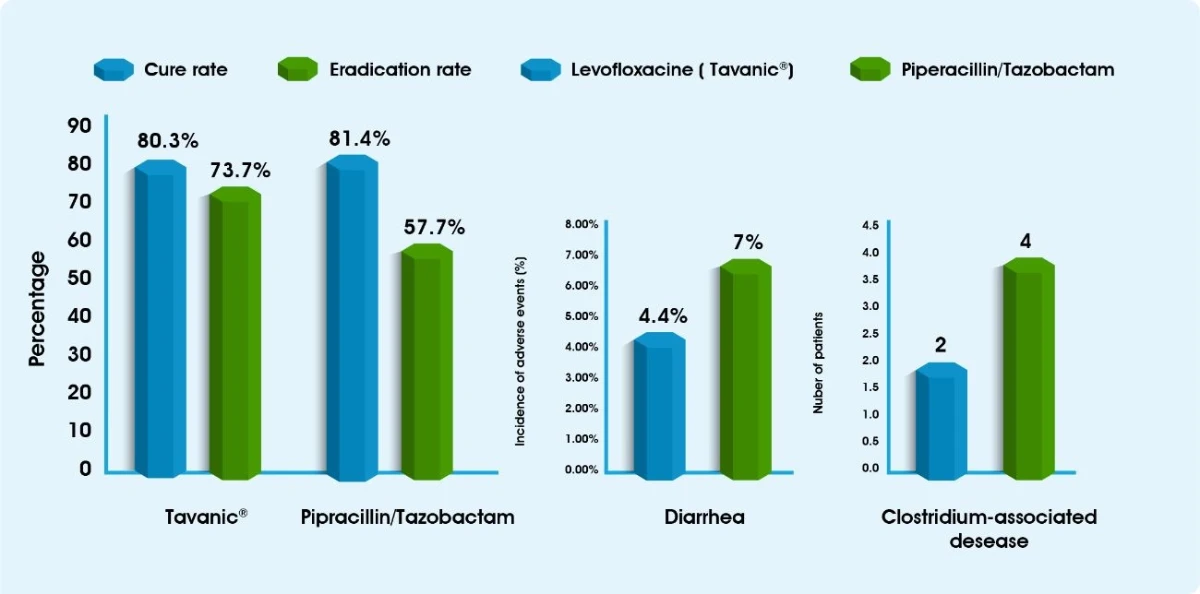
Hospital acquired pneumonia5
- JAC(2007) 60.605-612.
- cAsellas JM, Gilardoni M, et al. Comparitive in -vitro activity of levofloxacin against isolates of bacteria from adult patients with community -acquired lower respiratory tract infections. J Antimicrob Chemother 1999; 43 suppl.c;37-42.
- International Journal of Antimicrobial Agents 28S 2006; S115-S127.
- UM 2005; 25: 75-83.
- Infectious Diseases Society of America (IDSA). Levofloxacin (LVX) vs. Piperacillin/tazobactam (TZP) in the Treatment of Hospital-Acquired Pneumonia (HAP): A Prospective Randomized Study. Poster Session 2008. Available at: https://idsa.confex.com/idsa/2008/webprogram/ Paper25029.html #.Vk41yLIOoec.mailto. last accessed: 01.12.2015.
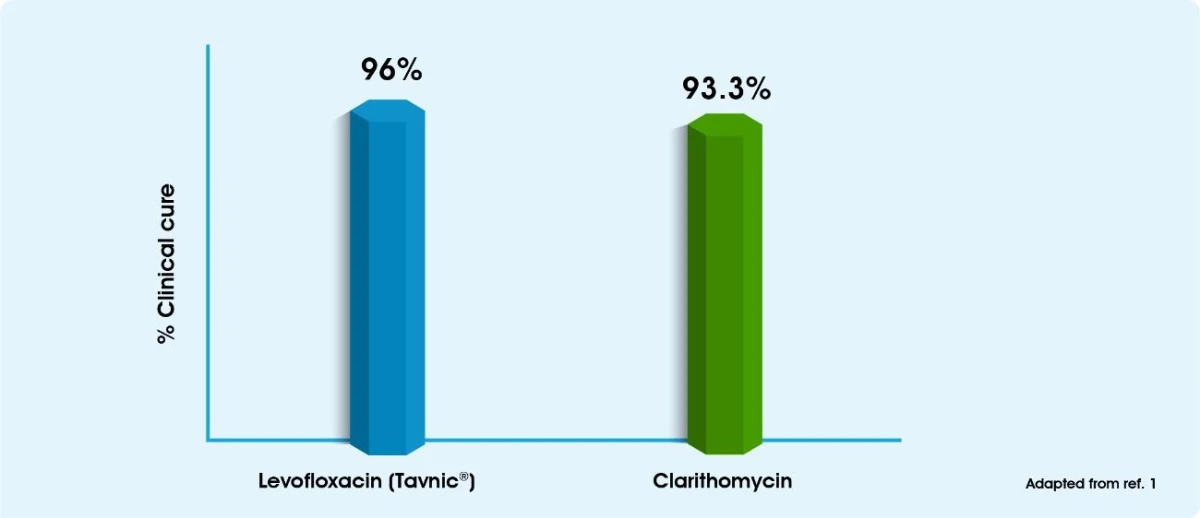
Efficacy & Safety of Tavanic® in the treatment of acute sinusitis1
Tavanic® in the management of DFI2
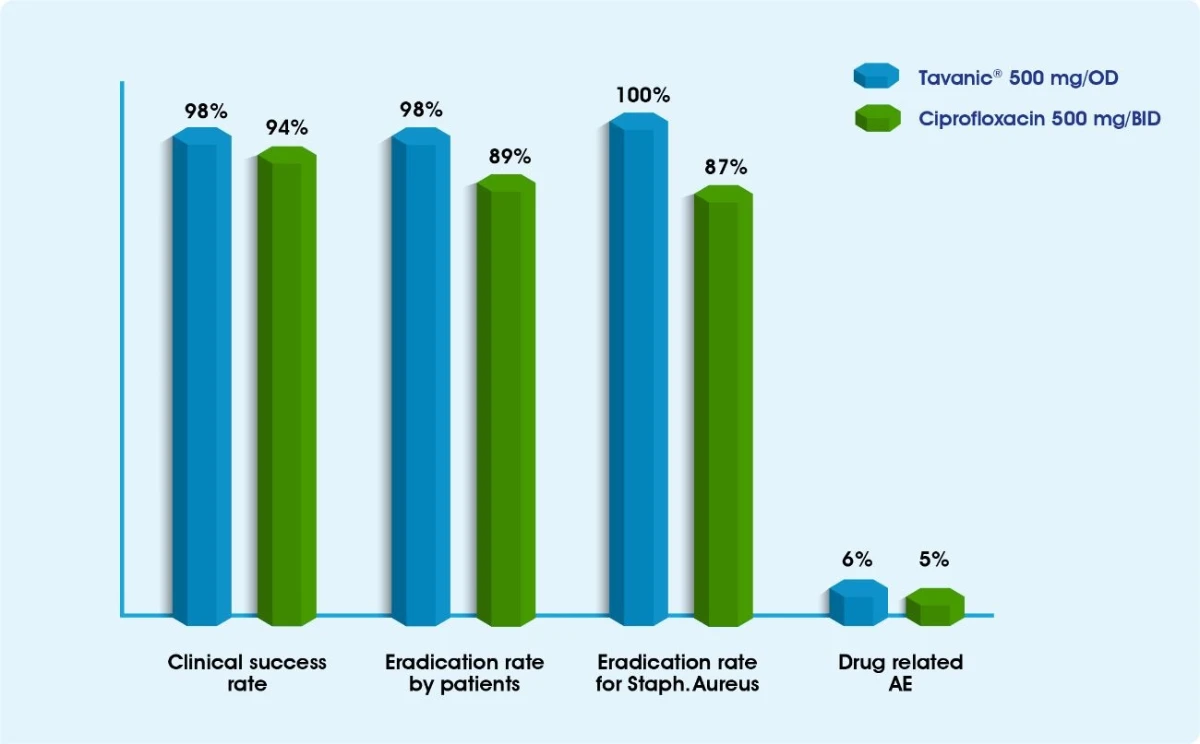
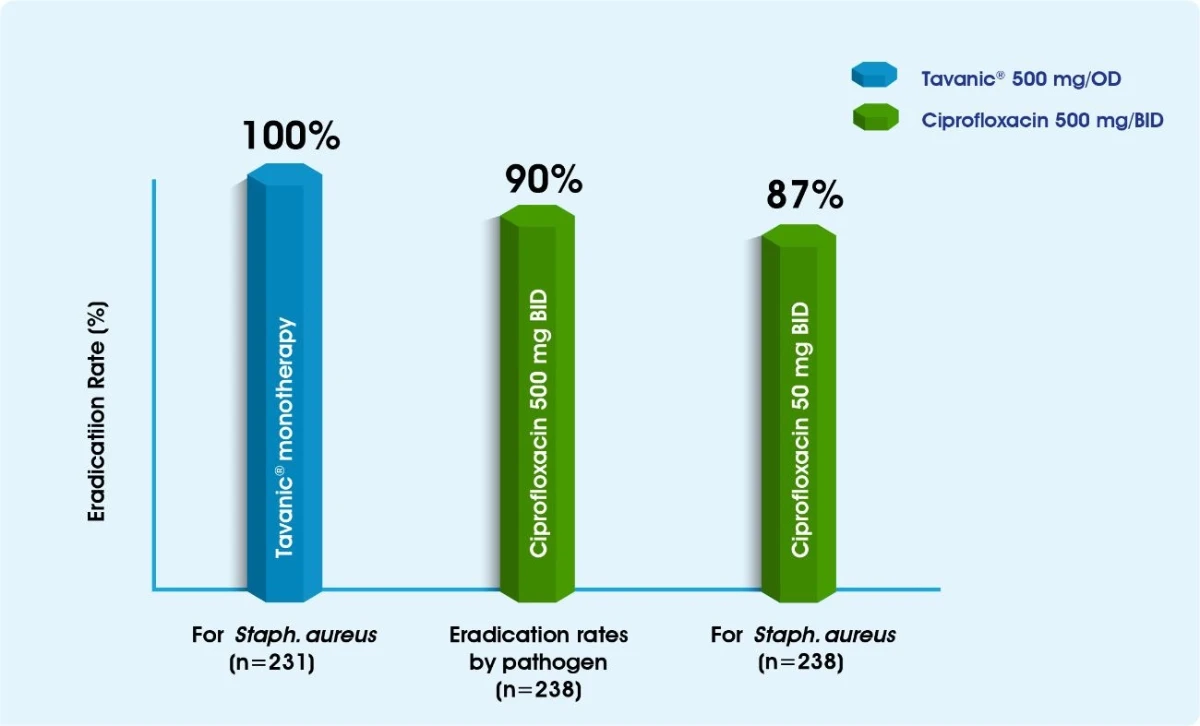
Tavanic® in the management of SSSI & bacteremia1-3
Tavanic® for SSSI Infections
Tavanic® has demonstrated excellent activity against the organisms most frequently encountered in SSSIs
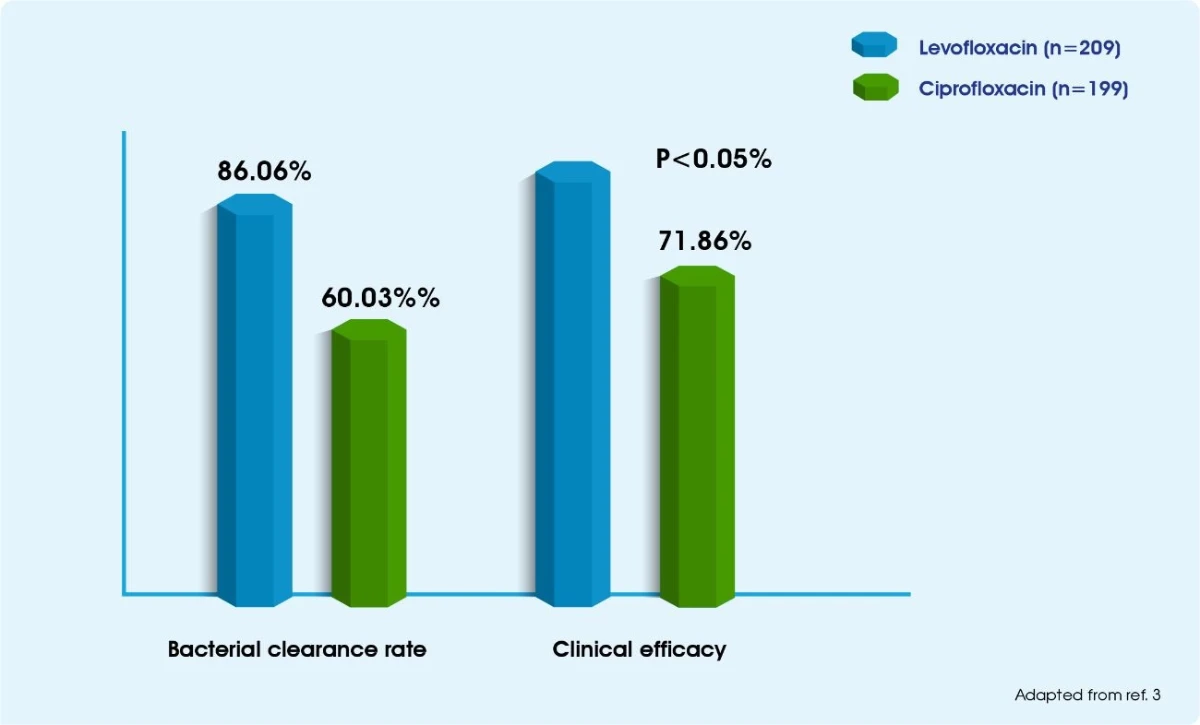
Tavanic effectively manages prostatitis3
Tavanic 500 mg is better than Ciprofloxacin in terms of bacterial clearance rate and resolution of clinical symptoms in chronic bacterial prostatitis
- South Med J. 1997; 90 (12): 1193-200.
- Nichols RL , Smith JW, Gentry LO, et al. Multicenter, randomized study comparing levofloxacin and ciprofloxacin for uncomplicated skin and skin structure infections. South Med J. 1997 Dec; 90(12): 1193-200.
- Zh i-chao Zhang, Feng-shuo Jin, et al. Safety and efficacy of levofloxacin versus ciprofloxacin for the treatment of chronic bacterial prostatitis in Chinese patients. Asian journal of Andrology 2012; 14: 870-87
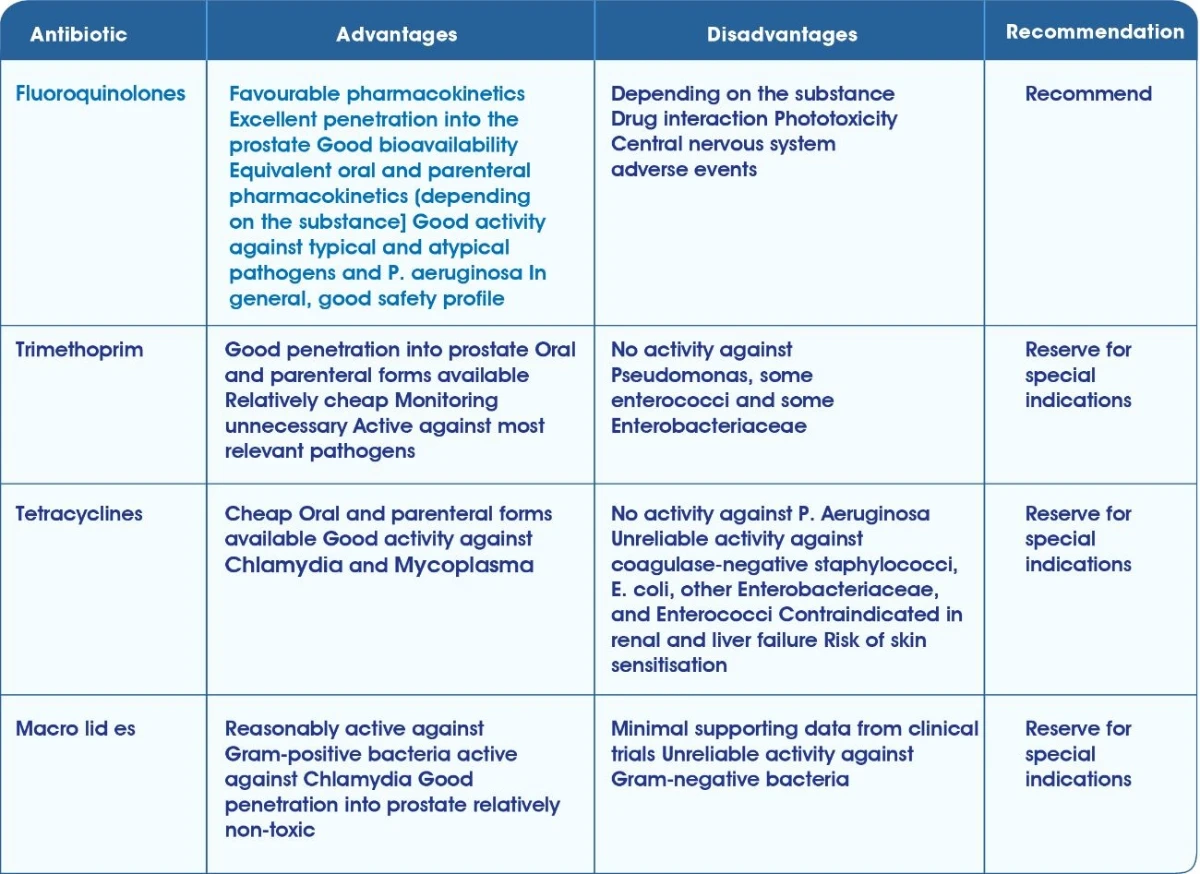
Guidelines
Guidelines for Empiric Antimicrobial Management of CAP1
Non-susceptibility rates of Streptococcus pneumoniae
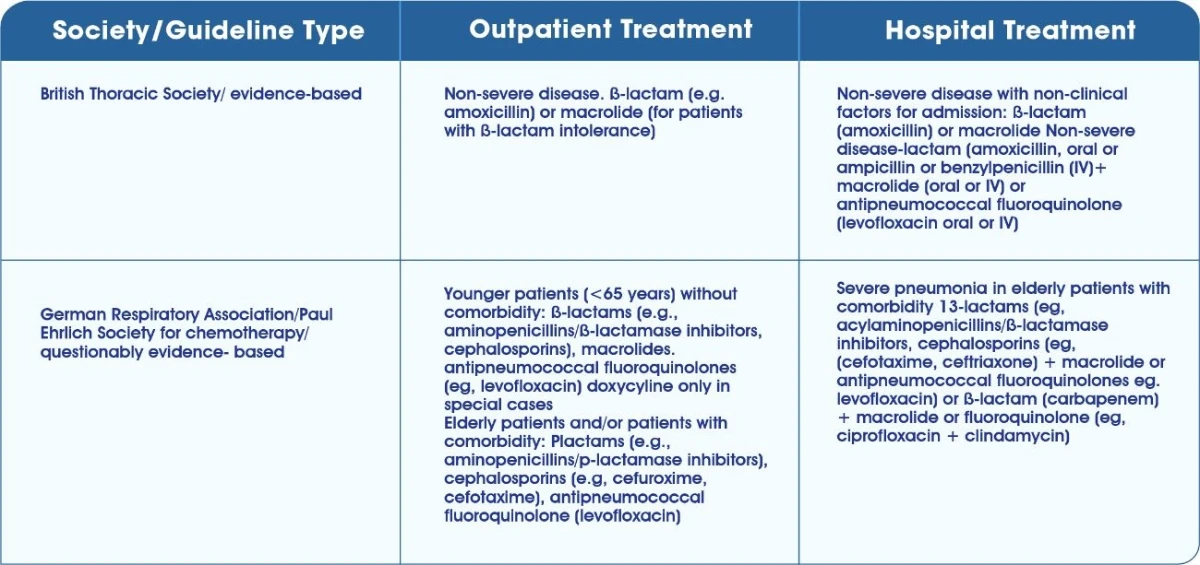
Guidelines for Empiric Antimicrobial Guidelines for the management of HAP,VAP and Healthcare associated pneumonia management of CAP2,3
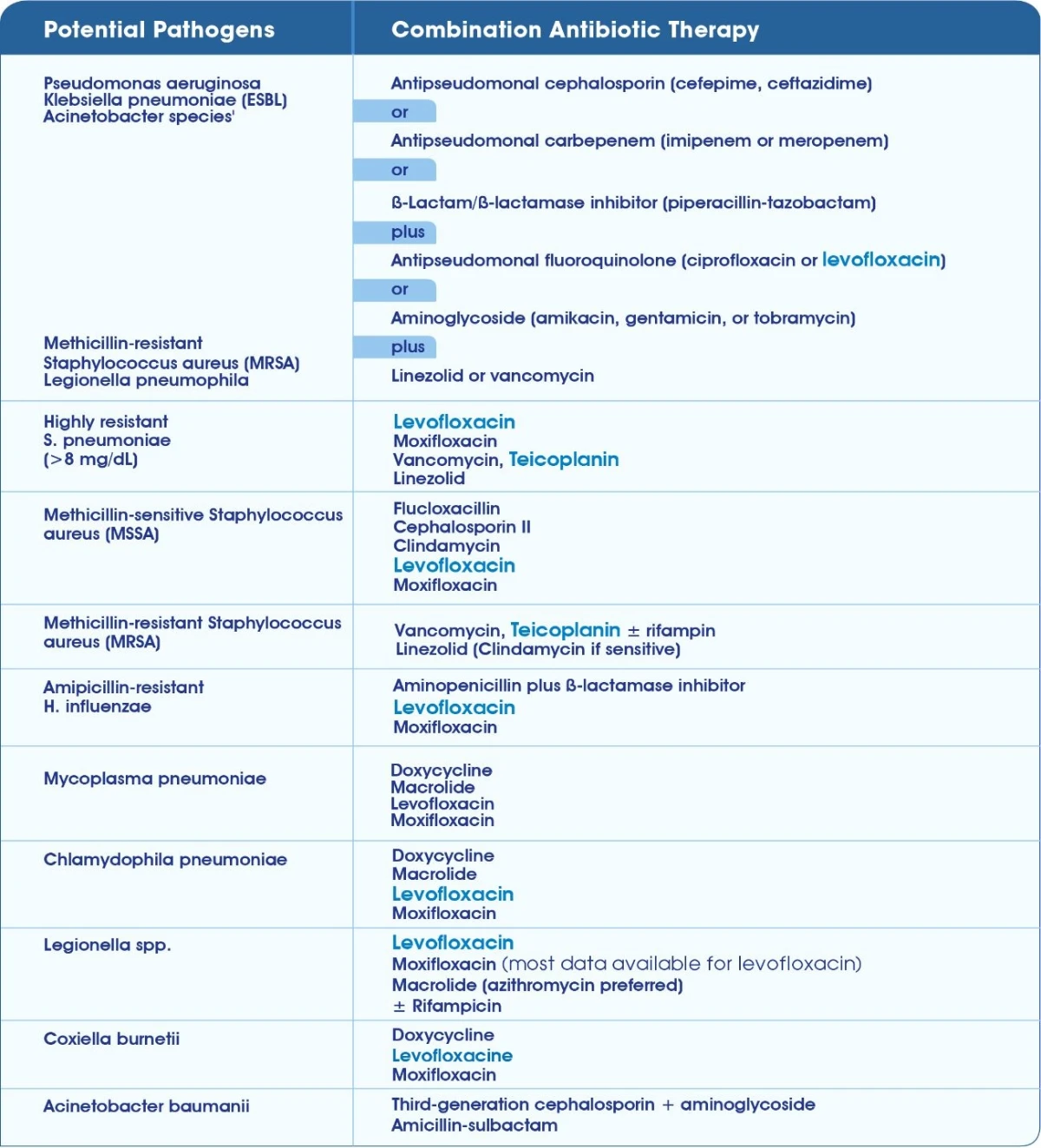
Anti-microbial treatment guidelines for the management of ABRS IDSA
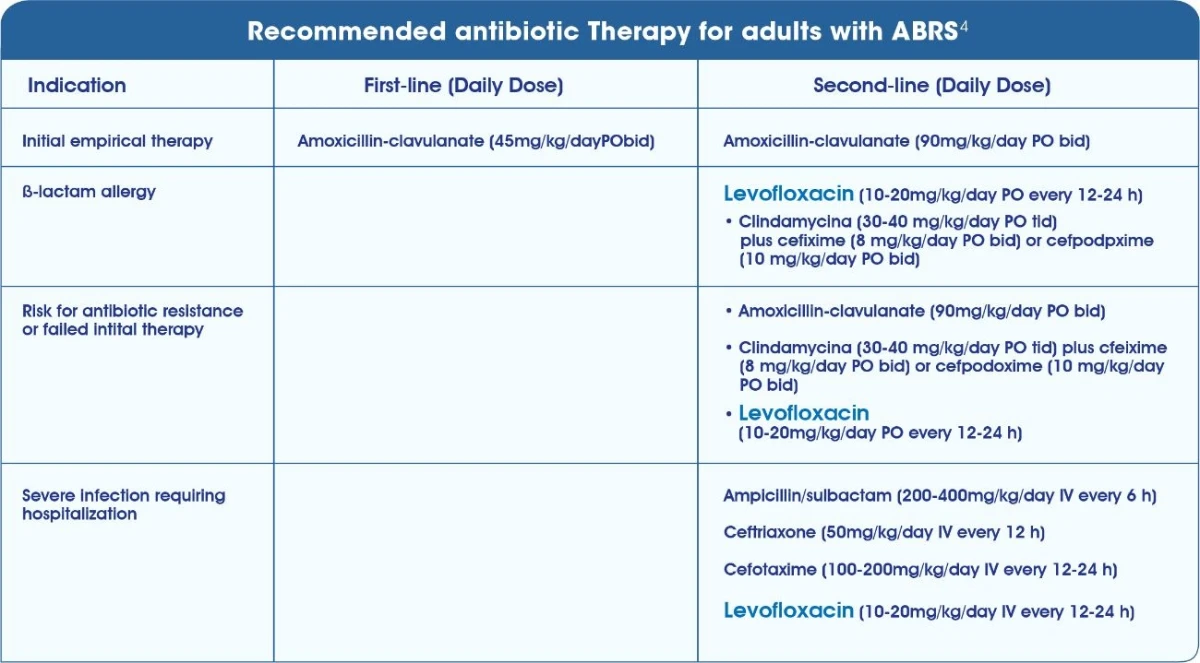
IDSA DFI5
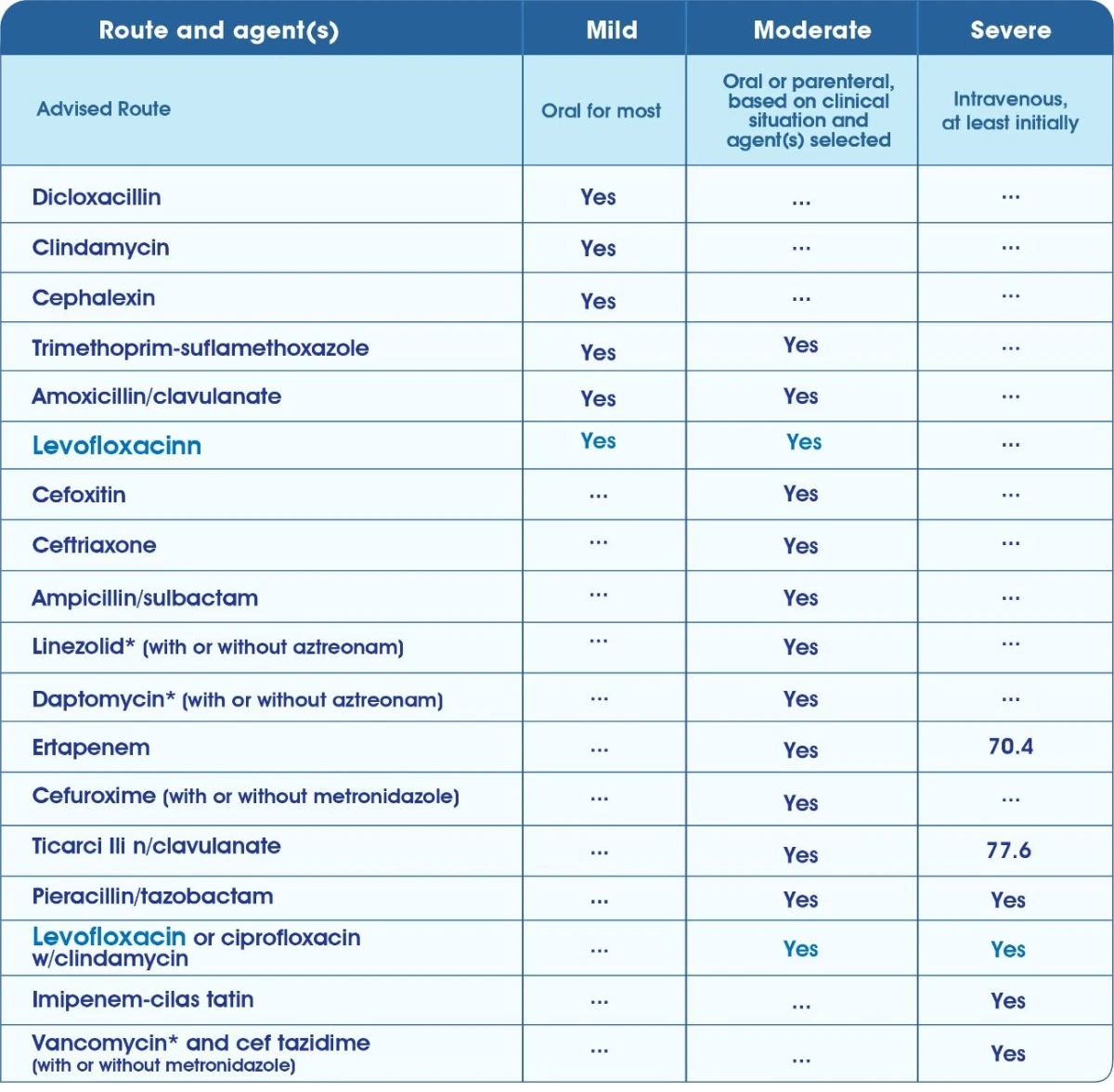
Recommended initial empiric oral antimicrobial therapy in mild & moderate acute uncomplicated pyelonephritis 6
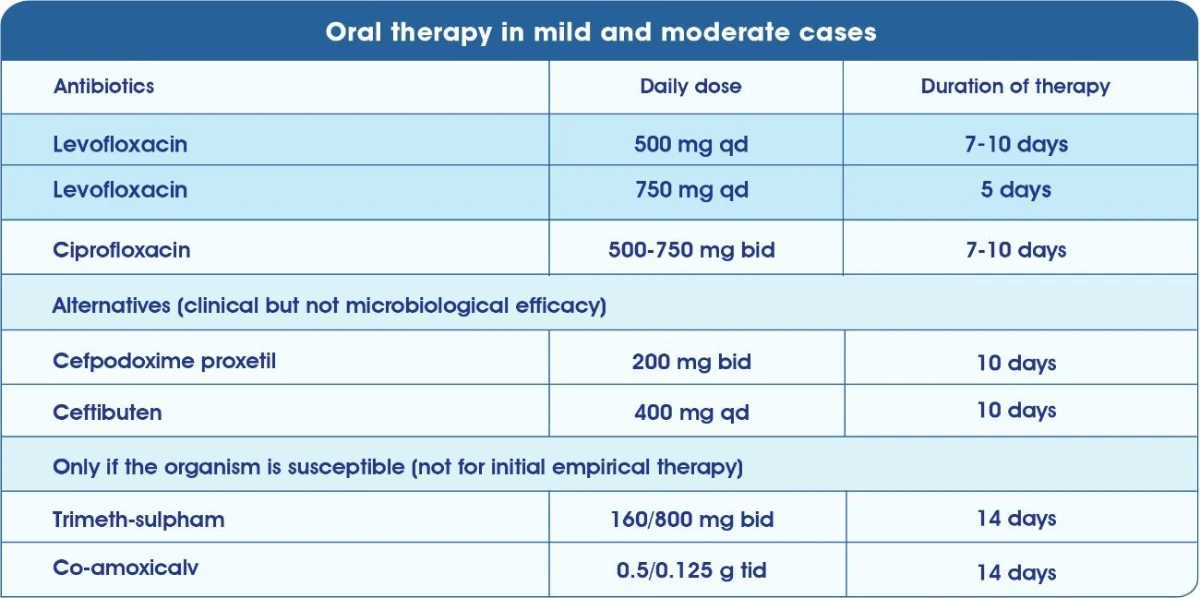
Initial parenteral dosing in severe cases6
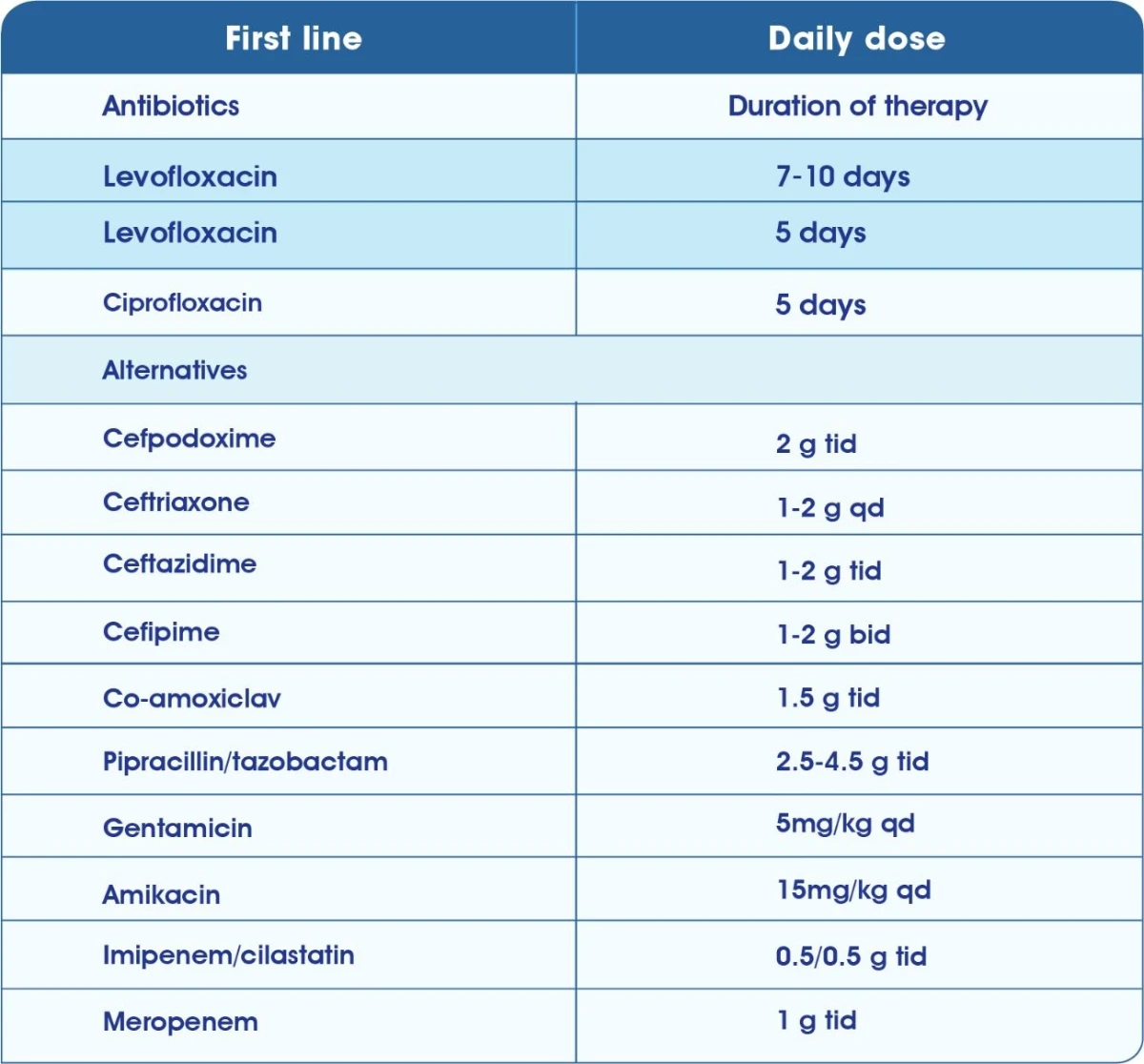
Treatment of Chronic bacterial prostatitis6
- File TM jr, Garau J, Blasi F, et al. Guidelines for empiric antimicrobial prescribing in community-acquired pneumonia. Chest 2004; 125 (5): 1888-901.
- American Thoracic society , Infectious Disease Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171 (4): 388-416.
- Woodhead M, Blasi F, Ewig 5. Guidelines for the management of adult lower respiratory tract infections - Summary. Clinical Microbiology and Infection 2011; 17 (Suppl. 6): 1-24.
- Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 2012; 54 (8): 72-112.
- Infectious Disease Society of America Guidelines for Diabetic Foot Infections CID 2004; 39: 885-910.
- EAU Guidelines 2015
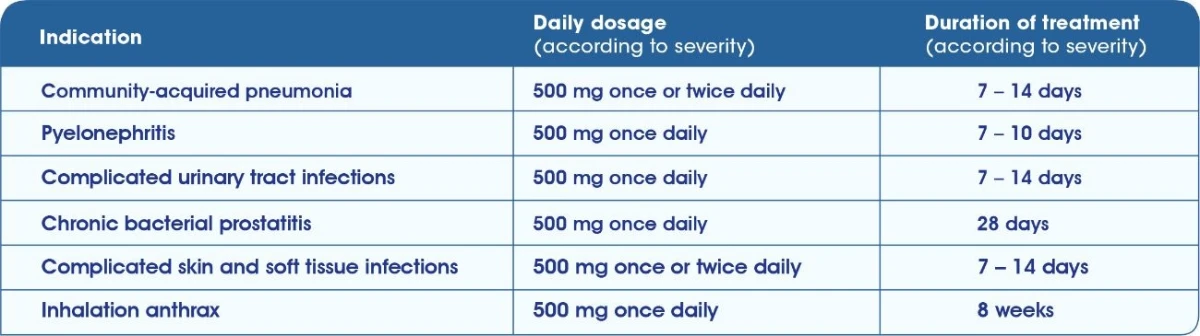
TAVANIC® Indications & Dosage1 Tavanic 500 mg Film tablet
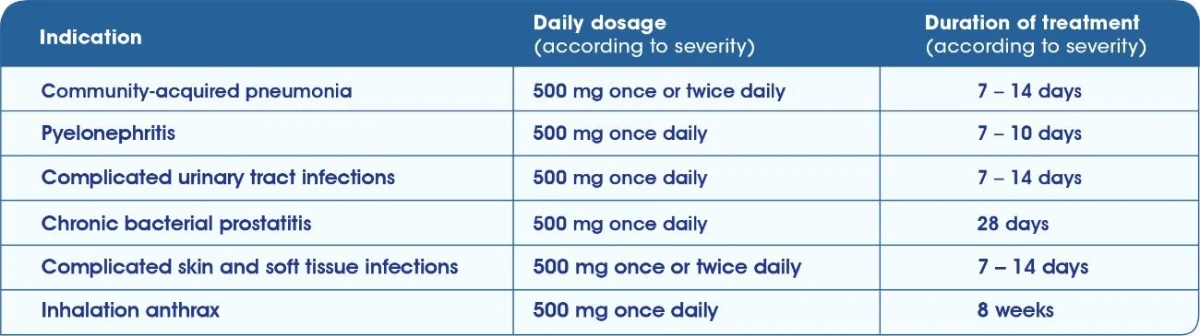
Tavanic® 5 mg/ml solution for infusion1
.webp)
Since Tavanic® may be administered either parenterally or orally a sequential timely conversion from IV to Oral therapies enables cost effective treatment & early hospital discharge2
- Tavanic SmPC March 2016
- Furlanut M, Brollo L, et al. Pharmacokinetic aspects of levofloxacin 500mg once dialy during sequential intravenous/oral therapy in patients with lower respiratory tract infections. J Antimicrob chemother 2003; 51 (1): 101-6.
TAVANIC® Summary
Tavanic® with Targeted Power
- Wide experience
- High Clinical Success rate1-3
• AECB • ABS • cUTIs • CAP • SSSIs • Prostatitis - Broad spectrum Coverage1
• Gram positive • gram negative • Atypical pathogens - Favourable PK/PD profile1
• Good tissue diffusion • Can be administered without regard to food • 99% bioavailability
• Sequential Therapy (oral IV) - Convenient OD dosing1
- Better safety profile4
- FDA approval of Levofloxacin. September 2008.
- 16th ECCMID April 2006, Abstract:pl 048.
- SouthMedj. 1997; 90 (12): 1193-200.
- Clinical Infectious disease 1999; 28: 352-64.
MAT-BH-2200538/V2/August 2022