
Efficacy
* The Power refers to the power of Rosuvastatin as a lipid lowering statin. As proven by the Stellar trial, rosuvastatin 10 to 40 mg was more efficacious in improving the overall lipid profile, non-HDL-C, apolipoproteins, and lipid ratios in patients with hypercholesterolemia than milligram equivalent doses of atorvastatin and milligram equivalent or higher doses of simvastatin and pravastatin.1
Confidence refers to the confidence in achieving lower LDL-C levels. The results from the ACTE trial showed that LDL cholesterol reduction and attainment of LDL cholesterol targets were significantly greater with the addition of ezetimibe 10 mg to stable rosuvastatin starting doses than was with rosuvastatin up-titration.²
- Jones PH, et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the Stellar trial. Clin Ther. 2004;26(9):1388-99.
- Bays HE, et al. Safety and efficacy of ezetimibe added on to rosuvastatin 5 or 10 mg versus up-titration of rosuvastatin in patients with hypercholesterolemia (the ACTE Study). Am J Cardiol. 2011;108(4):523-30.
A powerful combination for LDL-C reduction

HMG-CoA: hydroxy-methyl-glutaryl-coenzyme A; LDL-C: low density lipoprotein cholesterol
- Jones PH, et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on nonhigh-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia:additional results from the Stellar trial Clin Ther.2004;26(9):1388-99.
- Zympass SmPC November 2019.
- Krahenbuhl S, et al. Unmet needs in LDL-C lowering:when statins won’t do! Drugs. 2016;76(12):1175-90.
Zympass® is combining the most effective statin, Rosuvastatin to Ezetimibe:
Rosuvastatin produces larger reductions in LDL-C than other statins1
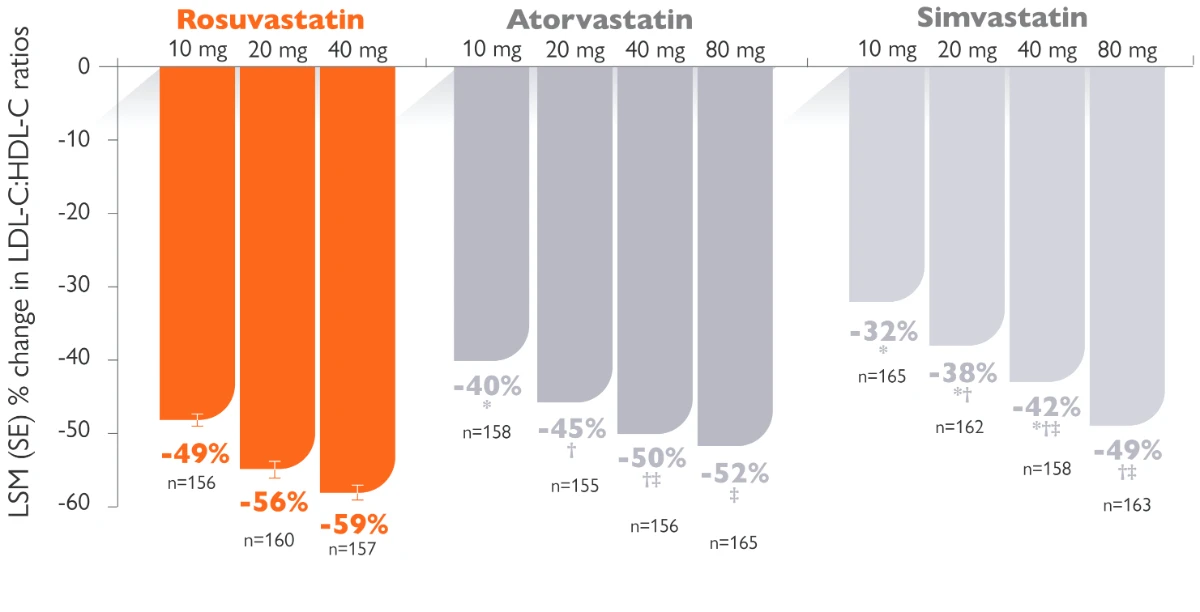
Randomized, multicentre, parallel-group, open-label trial. 2,268 patients ≥18 years of age received rosuvastatin 10, 20, 40, or 80 mg; atorvastatin 10, 20, 40, or 80 mg; simvastatin 10, 20, 40, or 80 mg; or pravastatin 10, 20, or 40 mg for 6 weeks.
The 40 mg dose of rosuvastatin should only be considered in patients with severe hypercholesterolaemia at high cardiovascular risk, and specialist supervision is recommended.
Objective: The goal of this study was to examine prospectively the effects of rosuvastatin, atorvastatin, simvastatin, and pravastatin across dose ranges on non-HDL-C, apo B, apo A-I, and total cholesterol (TC): HDL-C, low-density lipoprotein cholesterol (LDL-C): HDL-C, non-HDL-C: HDL-C, and apo B:apo A-I ratios in patients with hypercholesterolemia (LDL-C >160 mg/dL and <250 mg/dL and triglycerides <400 mg/dL) in the Statin Therapies for Elevated Lipid Levels compared Across doses to Rosuvastatin (STELLAR) trial.
LDL-C: low density lipoprotein cholesterol; LSM: least squares mean; SE: standard error
- Jones PH, et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the Stellar trial. Clin Ther. 2004;26(9):1388-99.
- Zympass SmPC November 2019
By adding ezetimibe to rosuvastatin 40 mg, approximately another 13 % LDL reduction was achieved:
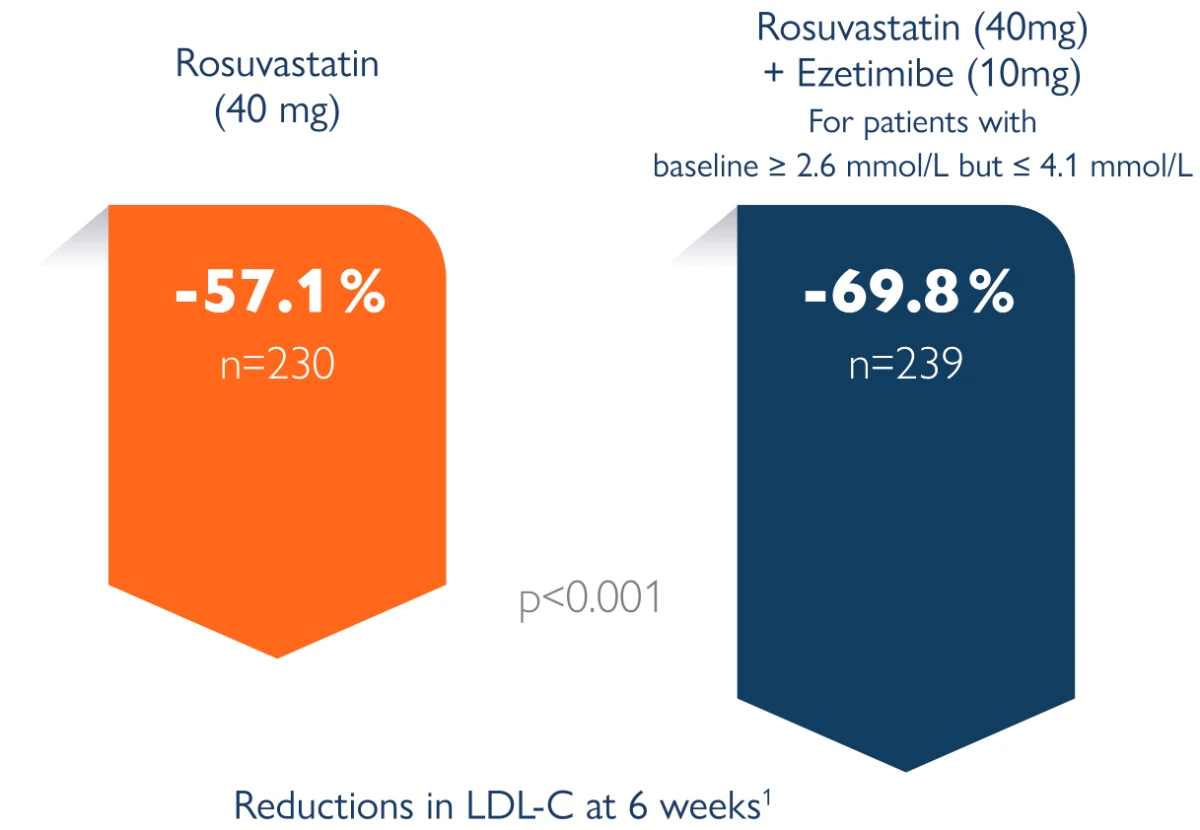
The aim of the EXamination of Potential Lipid modifying effects of Rosuvastatin in combination with Ezetibime versus Rosuvastatin alone (Explorer) study is to compare the efficacy and safety of the most effective and highest marketed dose of rosuvastatin (40 mg) alone or in combination with ezetibime 10 mg for 6 weeks in high-risk patients with hypercholestrolemia. (D3569C00006) is to compare the efficacy and safety of the most effective and highest marketed dose of rosuvastatin (40 mg) alone or in combination with ezetibime 10 mg for 6 weeks in high-risk patients with hypercholestrolemia.
Clinical study, open-label, with 469 patients randomly assigned to rosuvastatin alone (40 mg) or in combination with ezetimibe (40 mg/10 mg) for 6 weeks. Study conducted in 58 centres (USA, EU, South Africa). Explorer study1
The 40 mg / 10 mg dose is contraindicated in patients with pre-disposing factors for myopathy/ rhabdomyolysis. Such factors include: Moderate renal impairment (creatinine clearance <60 ml/ min), Hypothyroidism, Personal or family history of hereditary muscular disorders, Previous history of muscular toxicity with another HMG-CoA reductase inhibitor or fibrate, Alcohol abuse, Situations where an increase in plasma levels of rosuvastatin may occur and Asian patients.
Population : Men and women aged 18 years with hypercholesterolemia and a history of CHD or clinical evidence of atherosclerosis or a CHD risk equivalent (10-year CHD risk score 20%) were eligible for randomization if the mean of their 2 most recent fasting LDL cholesterol levels was 160 mg/dl and 250 mg/dl and the 2 measurements were within 15% of each other.
- Ballantyne CM, et al. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the Explorer study). Am J Cardiol. 2007;99(5):673-80
- Zympass SmPC November 2019
By adding ezetimibe to current rosuvastatin dose, a 15 % LDL reduction was achieved versus doubling the current dose of statin.
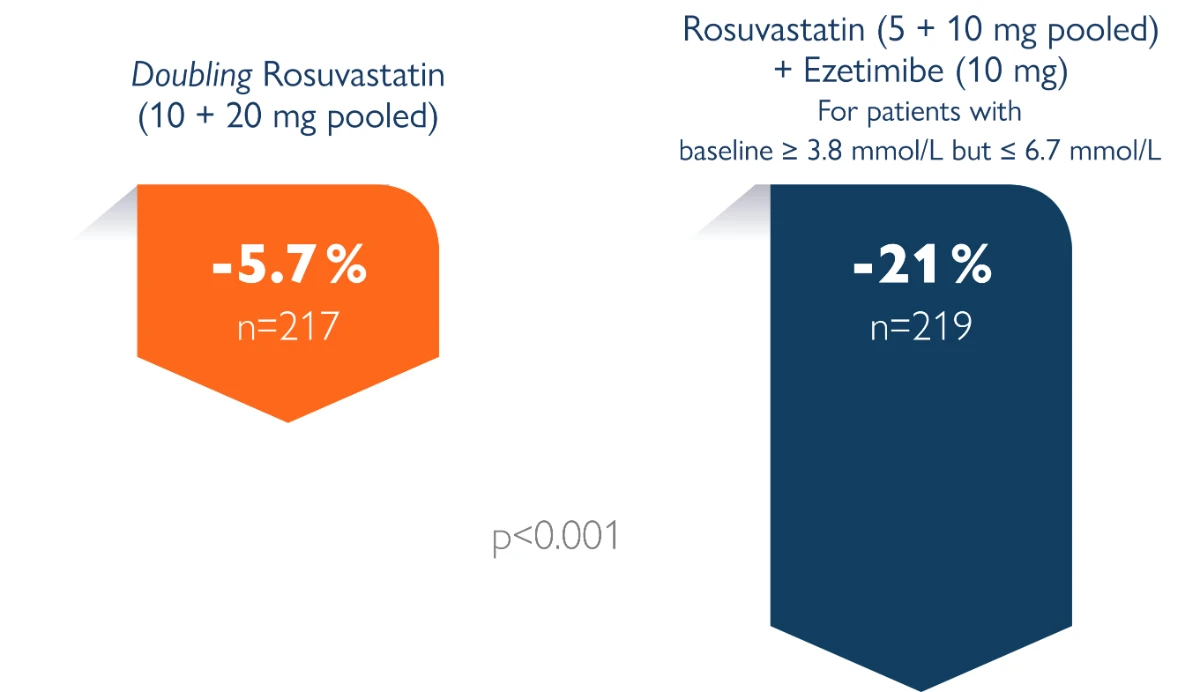
The primary objective of the present study was to compare the low-density lipoprotein (LDL) cholesterol-lowering efficacy of ezetimibe added to stable rosuvastatin therapy (5 or 10 mg/day, pooled across doses) versus doubling the rosuvastatin dose (from 5 to 10 mg/day or 10 to 20 mg/day, pooled across doses). The study population included hypercholesterolemic patients at moderately high or high risk of coronary heart disease (CHD) who were not at the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) optional LDL cholesterol levels of 100 mg/dl for moderately high or high-risk patients without atherosclerotic vascular disease (AVD) or 70 mg/dl for high-risk patients with AVD.1 The secondary objectives included comparison of the LDL cholesterol-lowering efficacy within each starting run-in rosuvastatin dose, attainment of NCEP ATP III-recommended LDL cholesterol targets of 70 mg/dl or 100 mg/dl, the percentage of change from baseline in other lipid parameters, and safety assessment.
Randomised, double-blind parallel group clinical study in 440 moderately high/high-risk patients randomly assigned to up-titration of rosuvastatin alone (from 5 to 10 mg or 10 to 20 mg) or rosuvastatin (5 to 10 mg) in combination with ezetimibe (10 mg) for 6 weeks. Study conducted in 57 centres in North America and Europe. ACTE study1
- Bays HE, et al. Safety and efficacy of ezetimibe added on to rosuvastatin 5 or 10 mg versus up-titration of rosuvastatin in patients with hypercholesterolemia (the ACTE Study). Am J Cardiol. 2011;108(4):523-30
Product Characteristics
- 10mg/10mg, 20mg/10mg and 40mg/10mg: Each film-coated tablet contains 10mg; 20mg or 40mg of rosuvastatin (as rosuvastatin calcium) respectively, and 10mg ezetimibe. Indication: Zympass is indicated for substitution therapy in adult patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently at the same dose level as in the fixed combination, but as separate products, as adjunct to diet for treatment of primary hypercholesterolemia (heterozygous familial and non-familial) or homozygous familial hypercholesterolemia.
-
SmPC, Date of Revision Nov 2019.
Safety Guideline
- Adding ezetimibe to rosuvastatin was not associated with worsening of side effects
Facilitating LDL-C goal achievement
...without worsening side effects
Number of patients with adverse events1
|
Variable |
Rosuvastatin 10, 20 mg (n=219) |
Rosuvastatin 5, 10 mg + Ezetimibe 10 mg (n=221) |
|
≥1 Event |
31 (14.2%) |
33 (14.9%) |
|
Drug-related‡ |
6 (2.7%) |
10 (4.5%) |
|
Serious drug-related‡ |
0 |
0 |
|
Discontinuations§ Drug-related‡ |
1 (0.5%) 0 |
5 (2.3%) 5 (2.3%) |
|
Deaths |
0 |
0 |
All AEs collected up to end of 14-day follow-up window were included in analysis.
‡ Determined by the investigator to be related to the drug; assessment of drug causality determined using following criteria: definitely, probably, possibly, probably not, and definitely not related.
§ Study medication withdrawn
The present multicenter, 6-week, randomized, double-blind, parallel-group, clinical trial evaluated the safety and efficacy of ezetimibe (10 mg) added to stable rosuvastatin therapy versus up titration of rosuvastatin from 5 to 10 mg or from 10 to 20 mg. The primary objective of the present study was to compare the low-density lipoprotein (LDL) cholesterol-lowering efficacy of ezetimibe added to stable rosuvastatin therapy (5 or 10 mg/day, pooled across doses) versus doubling the rosuvastatin dose (from 5 to 10 mg/day or 10 to 20 mg/day, pooled across doses). The study population included hypercholesterolemic patients at moderately high or high risk of coronary heart disease (CHD) who were not at the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) optional LDL cholesterol levels of 100 mg/dl for moderately high- or high-risk patients without atherosclerotic vascular disease (AVD) or 70 mg/dl for high-risk patients with AVD.1 The secondary objectives included comparison of the LDL cholesterol lowering efficacy within each starting run-in rosuvastatin dose, attainment of the NCEP ATP III-recommended LDL cholesterol targets of 70 mg/dl or 100 mg/dl, the percentage of change from baseline in other lipid parameters, and a safety assessment.
- Ballantyne CM, et al. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the Explorer study). Am J Cardiol. 2007;99(5):673-80.
Usage
Adherence
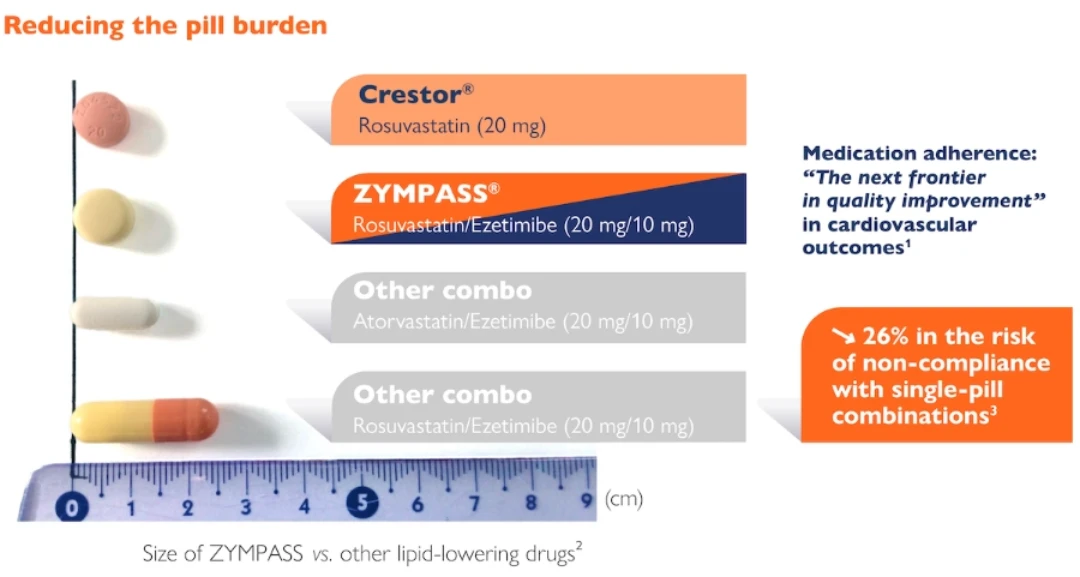
Zympass® is a small, easy to swallow statin – ezetimibe combination
- Ho PM, et al. Medication adherence: its importance in cardiovascular outcomes; Circulation. 2009.
- Zympass SmPC.
- Bangalore S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713-9.
Zympass® reduces the pill burden on patients:
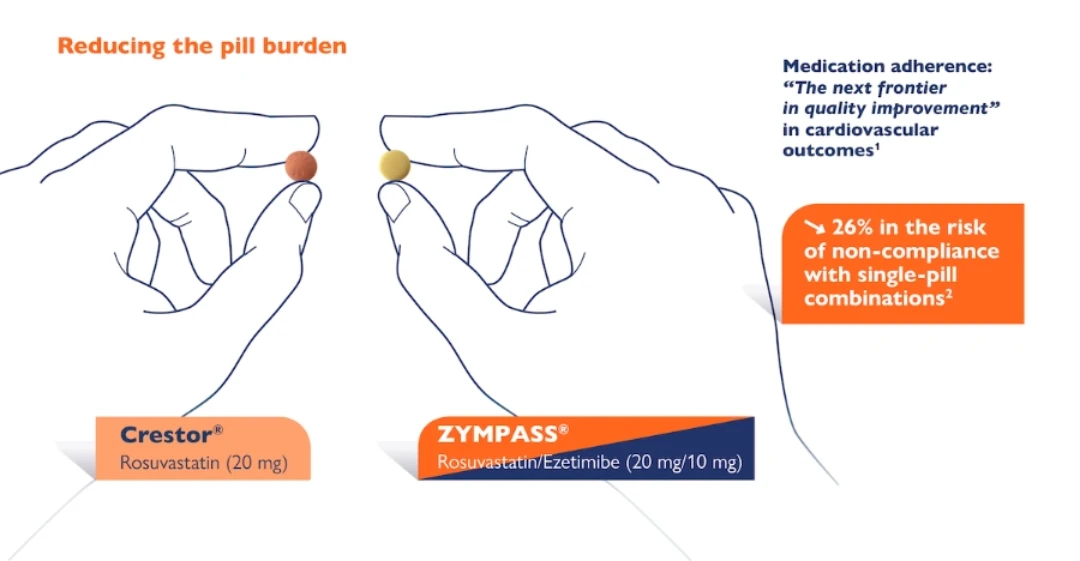
- Ho PM, et al. Medication adherence: its importance in cardiovascular outcomes; Circulation. 2009 16;119(23):3028-35.
- Bangalore S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713-9.
Usage Guideline
Zympass® offers a flexibility of a full dose range
Zympass®, the first single-pill combination available in 3 doses*

- Zympass SmPC November 2019.
