IL-4 and IL-13 are key and central cytokines with unique and overlapping roles contributing to the pathophysiology of CRSwNP
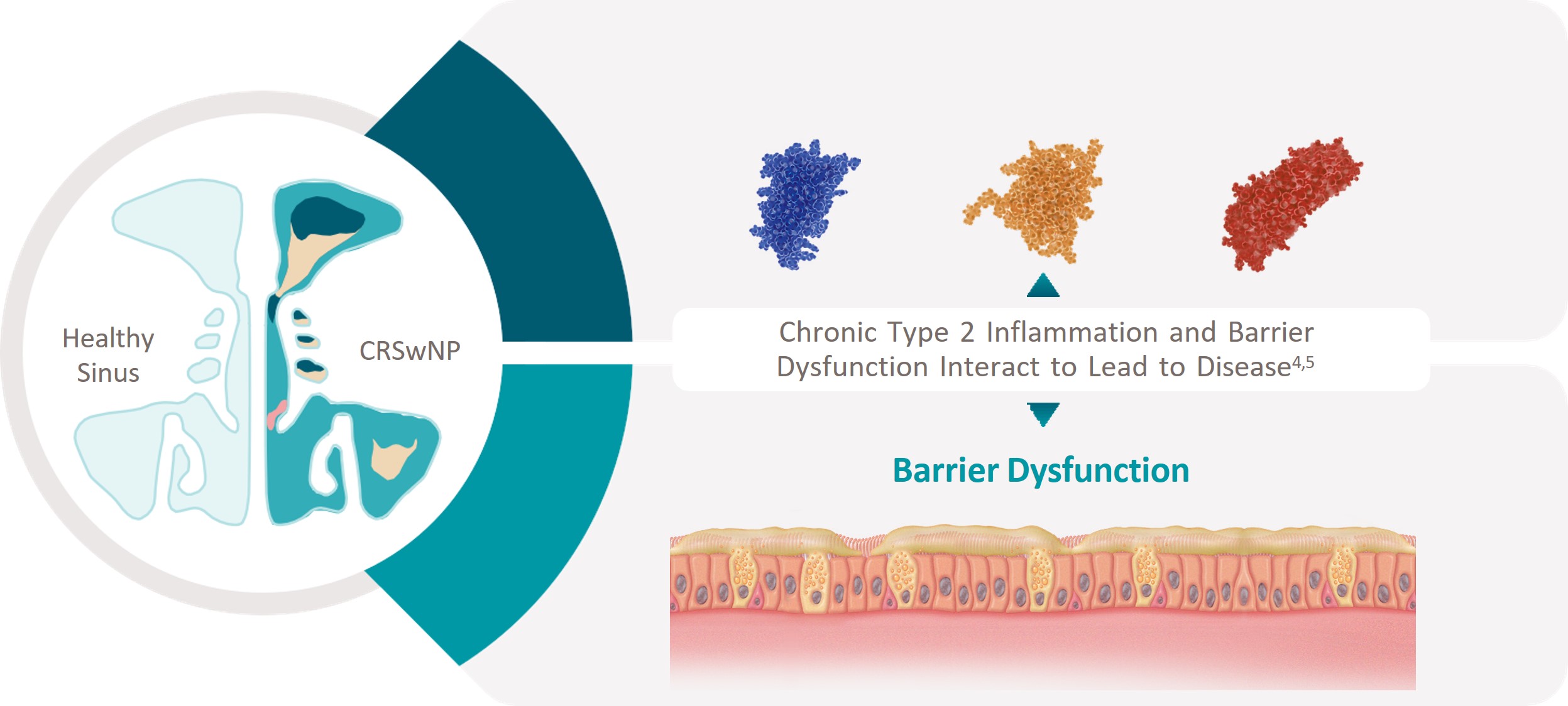
TYPE 2 INFLAMMATION UNDERLIES THE
PATHOPHYSIOLOGY OF CRSwNP
Up to 87% of CRSwNP Patients Have Type 2 Inflammation1,2
“…it is likely that patients with uncontrolled type 2 CRSwNP will increasingly
be treated with biologics” that target the underlying inflammation13
*May include antibiotics when infection is present, intranasal corticosteroid (consider increased dose), SCS, and/or nasal irrigation. CRSwNP, chronic rhinosinusitis with nasal polyps; IL, interleukin; SCS, systemic corticosteroids.
1. Stevens WW, et al. J Allergy Clin Immunol Pract. 2019;7(8):2812-2820.e3. 2. Orlandi RR, et al. Int Forum Allergy Rhinol. 2021;11(3):213-739. 3. Schleimer RP. Annu Rev Pathol. 2017;12:331-357. 4. Schleimer RP, Berdnikovs S. J Allergy Clin Immunol. 2017;139(6):1752-1761. 5. Soyka MB, et al. J Allergy Clin Immunol. 2012;130(5):1087-1096. 6. Turner JH, et al. Int Forum Allergy Rhinol. 2018;8(10):1175-1183. 7. De Corso E, et al. J Pers Med. 2022;12(8):1251. 8. Mullol J, et al. J Allergy Clin Immunol Pract. 2022;10(4):1086-1095.e5. 9. Fokkens WJ, et al. Rhinol Suppl. 2012;23:1-298. 10. Alobid I, et al. J Investig Allergol Clin Immunol. 2011;21 Suppl 1:1-58. 11. DeConde AS, et al. Laryngoscope. 2017;127(3):550-555. 12. Xian M, Zhang L. Allergy. 2023;78(3):623-625. 13. Bachert C, et al. Nat Rev Dis Primers. 2020;6(1):86.
Despite Geographical Heterogeneity, Type 2
Is the Most Prevalent Endotype in CRSwNP1-3
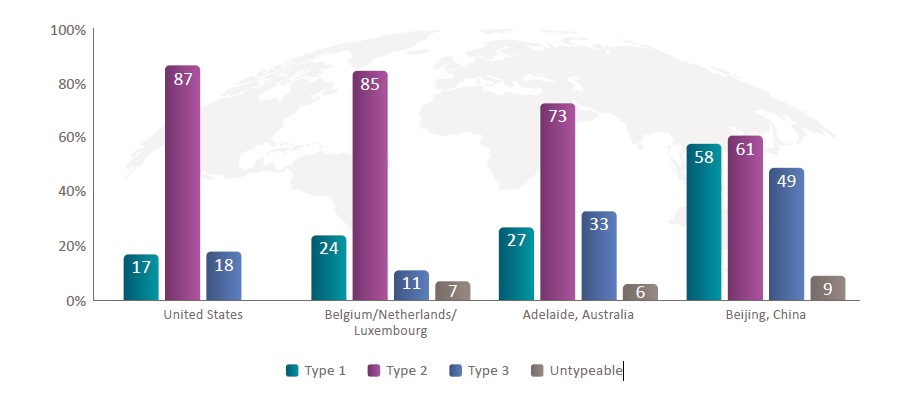
CRSwNP, chronic rhinosinusitis with nasal polyps.
1. Stevens WW, et al. J Allergy Clin Immunol Pract. 2019;7(8):2812-2820.e3. 2. Orlandi RR, et al. Int Forum Allergy Rhinol. 2021;11(3):213-739. 3. Staudacher AG, et al. Ann Allergy Asthma Immunol. 2020;124(4):318-325.
Key Type 2 Cytokines in CRSwNP1-6
Key Type 2 Cytokines
Pathophysiological Features
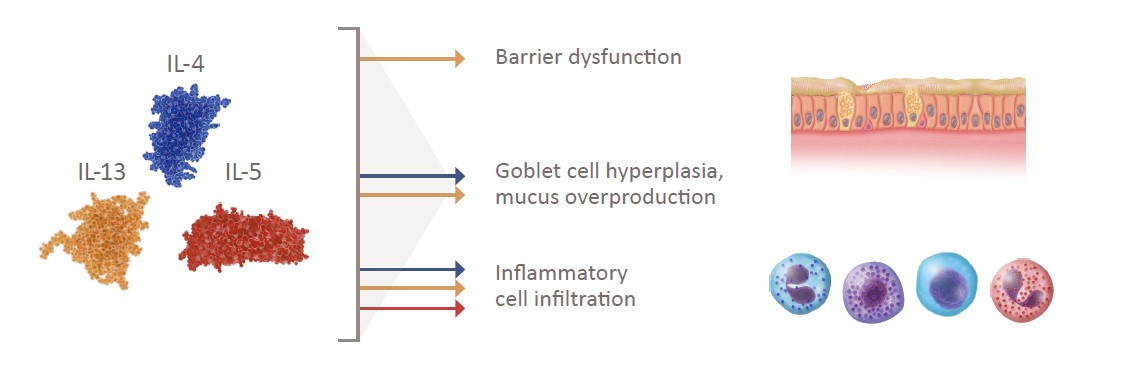
CRSwNP, chronic rhinosinusitis with nasal polyps; IL, interleukin.
1. Schleimer RP. Annu Rev Pathol. 2017;12:331-357. 2. Kato A. Allergol Int. 2015;64(2):121-130. 3. Gandhi NA, et al. Nat Rev Drug Discov. 2016;15(1):35-50. 4. Wise SK, et al. Int Forum Allergy Rhinol. 2014;4(5):361-370. 5. Takabayashi T, et al. J Allergy Clin Immunol. 2013;132(3):584-592.e4. 6. Chalermwatanachai T, et al. Sci Rep. 2018;8(1):7926.
Type 2 Inflammation and Barrier Disruption Interact
to Promote Further Damage1-10
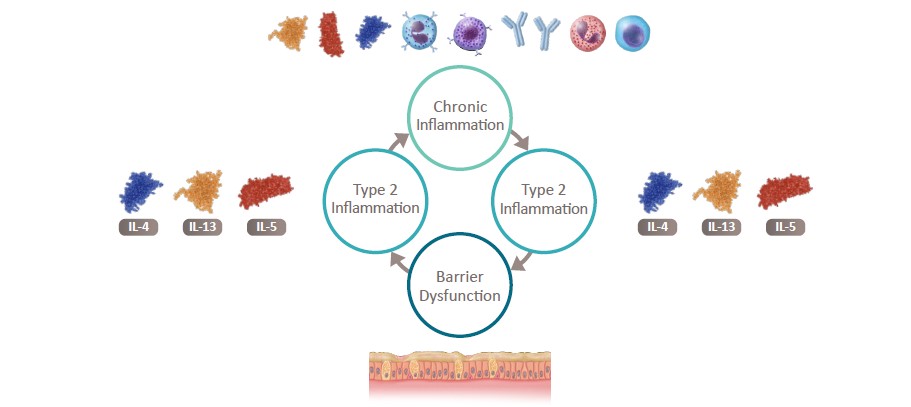
IL, interleukin.
1. Gandhi NA, et al. Nat Rev Drug Discov. 2016;15(1):35-50. 2. Schleimer RP. Annu Rev Pathol. 2017;12:331-357. 3. Ahern S, Cervin A. Medicina (Kaunas). 2019;55(4):95. 4. Kato A, et al. Chest. 2019;156(1):141-149. 5. Hulse KE, et al. Clin Exp Allergy. 2015;45(2):328-346. 6. Stevens WW, et al. Am J Respir Crit Care Med. 2015;192(6):682-694. 7. Zhai GT, et al. Laryngoscope. 2019;129(3):E110-E117. 8. Takabayashi T, et al. J Allergy Clin Immunol. 2013;132(3):584-592.e4. 9. Schleimer RP, Berdnikovs S. J Allergy Clin Immunol. 2017;139(6):1752-1761. 10. Soyka MB, et al. J Allergy Clin Immunol. 2012;130(5):1087-1096.e10.
Type 2 Cytokines Correlates With Clinical Presentation1,2
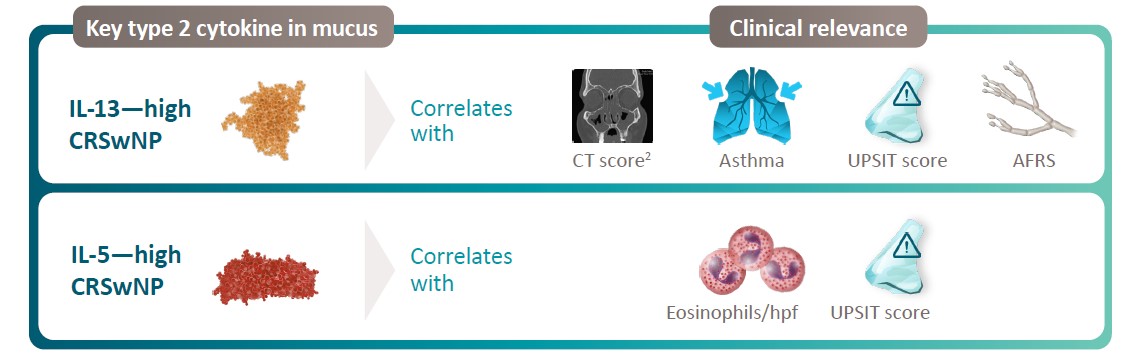
Patients with elevated IL-4 and IL-5 in mucus have higher SNOT-22* scores and more surgeries3†
*P value of t-test comparison between the groups <0.01. †P value of t-test comparison between the groups <0.05. AFRS, allergic fungal rhinosinusitis; CRSwNP, chronic rhinosinusitis with nasal polyps; CT, computed tomography; hpf, high-power field; IL, interleukin; UPSIT, University of Pennsylvania Smell Identification Test; SNOT-22, 22-item Sino-Nasal Outcome Test. 1. Turner JH, et al. Int Forum Allergy Rhinol. 2018;8(10):1175-1183. 2. Schleimer RP. Annu Rev Pathol. 2017;12:331-357. 3. De Corso E, et al. J Pers Med. 2022;12(8):1251.
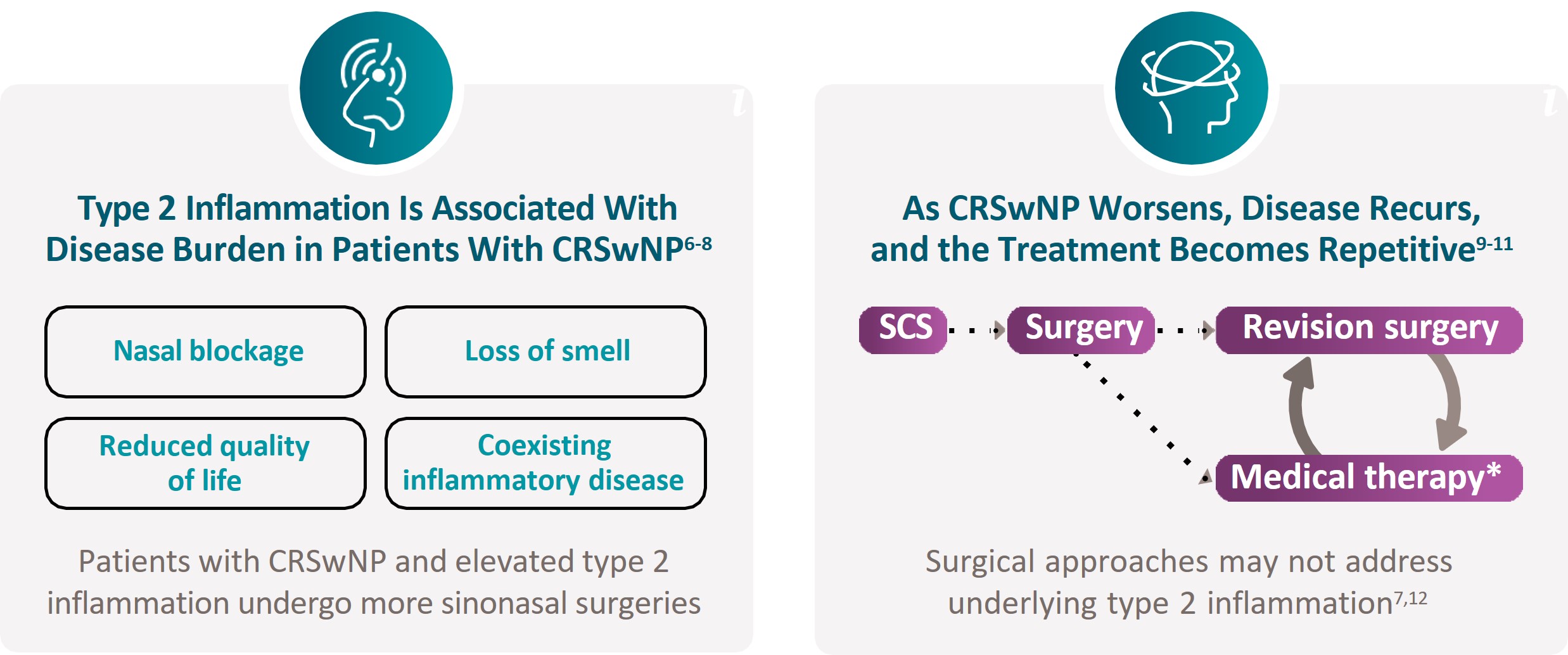
Limitations of CRSwNP Management
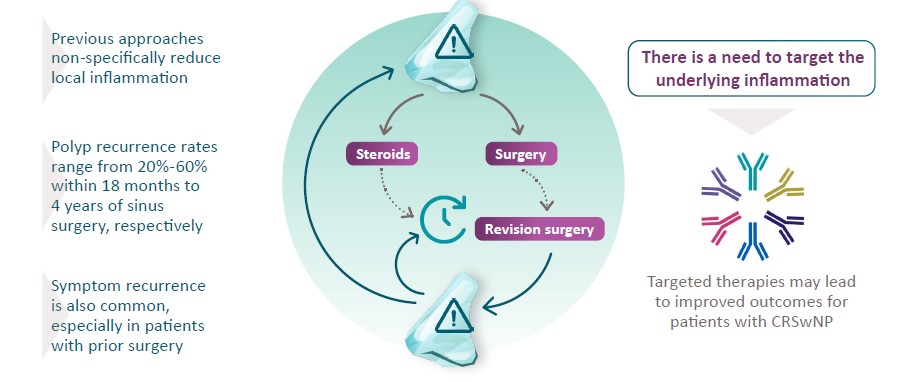
IL, interleukin.
Bachert C, et al. J Asthma Allergy. 2021;14:127-134.
