About Type 2 Inflammation – Atopic Dermatitis
.png/jcr:content/science%20hero%20(1).png)
The Continuous burden of Atopic Dermatitis can have far-reaching Impact
The burden of inadequately controlled atopic dermatitis may become chronic, or even cumulative, impacting patients throughout their lives1,2

~70 - 80% of atopic dermatitis patients develop additional type 2 inflammatory diseases, such as asthma or allergic rhinitis, which correlate with atopic dermatitis severity5,6
*The age of children included in the Eczema Society of Canada Atopic Dermatitis Quality of Life Report survey ranged from 0 to 18 years

~70 - 80% of atopic dermatitis patients develop additional type 2 inflammatory diseases, such as asthma or allergic rhinitis, which correlate with atopic dermatitis severity5,6
* The age of children included in the Eczema Society of Canada Atopic Dermatitis Quality of Life Report survey ranged from 0 to 18 years

~70 - 80% of atopic dermatitis patients develop additional type 2 inflammatory diseases, such as asthma or allergic rhinitis, which correlate with atopic dermatitis severity5,6
* The age of children included in the Eczema Society of Canada Atopic Dermatitis Quality of Life Report survey ranged from 0 to 18 years
- Zuberbier T et al. J Allergy Clin Immunol 2006; 118:226–232.
- Ibler K and Jemec GBE. Dermatol Rep 2011; 31; 3(1):8–10.
- Eczema Society of Canada. Atopic Dermatitis Quality of Life Report. 2017.
- Stocker RPJ et al. Arch Clin Neuropsychol 2017; 32:349–368.
- Davis DM et al. Semin Cutan Med Surg 2017; 36(3):95–99.
- Eckert L et al. Global Aware. Report Global 2018.
- Holm EA et al. J Eur Acad Dermat Venereol 2006; 20(3):255–259.
Around 3 in 5 adult Moderate-to-Severe Atopic Dermatitis patients are Inadequately controlled despite treatment1*
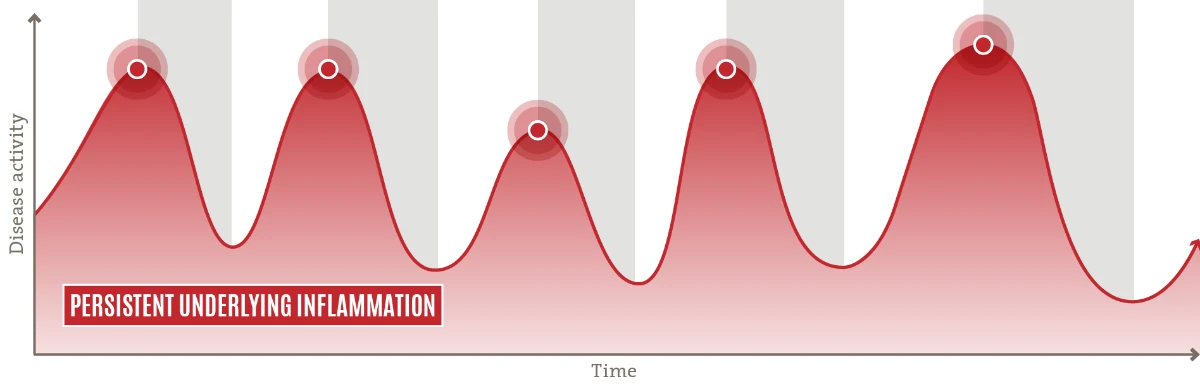
![]() A chronic, inadequately controlled disease driven by persistent underlying inflammation7,8
A chronic, inadequately controlled disease driven by persistent underlying inflammation7,8
![]() Patients may experience intensified disease activity for 30–50% of the year2
Patients may experience intensified disease activity for 30–50% of the year2
![]() Current treatment paradigm (OCS/ immunosuppressants) primarily addresses disease manifestation using a reactive, episodic approach9
Current treatment paradigm (OCS/ immunosuppressants) primarily addresses disease manifestation using a reactive, episodic approach9
Length of treatment with systemic steroids and other immunosuppressants is limited by common side effects and chance of rebounds11
Continuous treatment of underlying Inflammation is warranted for Long-term disease control10
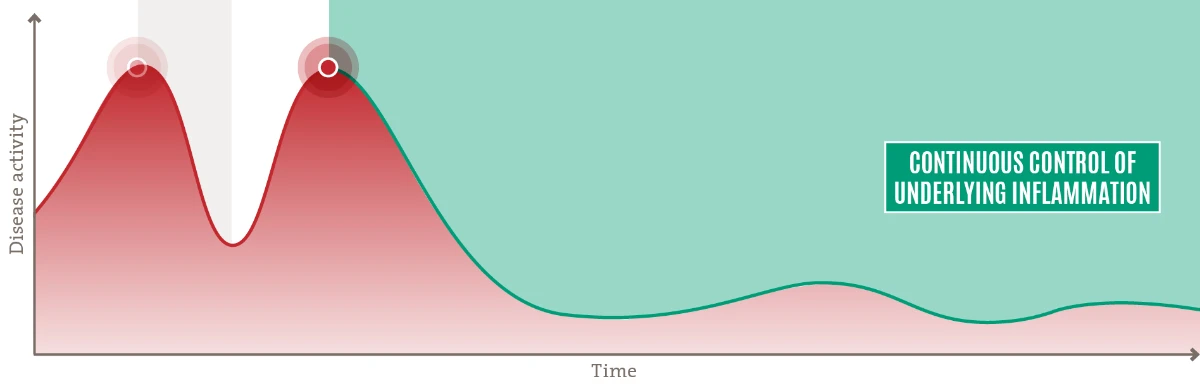
 A more sustainable treatment paradigm proactively targets the persistent underlying inflammation with a safety profile that allows for continuous treatment10-12
A more sustainable treatment paradigm proactively targets the persistent underlying inflammation with a safety profile that allows for continuous treatment10-12
 Adequately controlled disease with long-term improvements in signs, symptoms, and quality of life.
Adequately controlled disease with long-term improvements in signs, symptoms, and quality of life.
- Wei W et al. J Dermatol 2018; 45:150–157.
- Zuberbier T et al. J Allergy Clin Immunol 2006; 118:226–232.
- International Alliance of Dermatology Patient Organizations. Atopic Dermatitis: A Collective Global Voice for Improving Care. February 2018.
- Hajar T et al. An Bras Dermatol 2018; 93:104–107.
- Boguniewicz M et al. J Allergy Clin Immunol Pract 2017; 5:1519–1531.
- Ibler K & Jemec GBE. Dermatol Rep 2011; 3:e5.
- Gittler J et al. J Allergy Clin Immunol 2012; 130:1344–1354.
- Guttman-Yassky E et al. J Allergy Clin Immunol 2011; 127:1420–1432.
- Leung DYM et al. J Clin Invest 2004; 113:651–657.
- Wollenberg A et al. Ann Dermatol 2012; 24(3):253–260.
- Wollenberg A et al. J Eur Acad Dermatol Venereol 2018; 32(6):850–878.
- Wollenberg A et al. J Eur Acad Dermatol Venereol 2018; 32:657–682.
Dysregulation of the Type 2 Immune Response pathway leads to Underlying Type 2 Inflammation in Atopic Dermatitis and other Type 2 Inflammatory diseases*
Inflammatory Pathway | Type 1 | Type 2 | Type 3 |
| Primary immune cells1-3 |  | 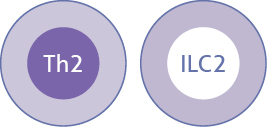 |  |
| Key cytokines1-4 | 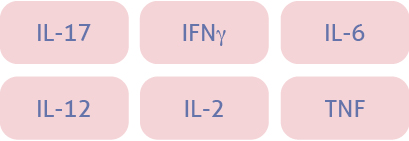 | 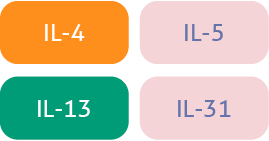 | 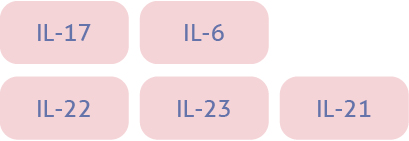 |
| Associated intracellular signaling pathway4,5 |  | .jpg) | .jpg) |
| Natural defensive roles against:1,3,6 | Viral infection Intracellular bacterialinfection Cancer cells | Allergens Parasites | Extracellular bacteria Fungi |
| Associated diseases when dysregulated7-8 | Psoriasis Psoriatic arthritis | Atopic dermatitis Asthma CRSwNP | Psoriasis Psoriatic arthritis |
*Simplified depiction based on key published information; not meant to be exhaustive in nature.
- Kaiko GE et al. Immunol 2008; 123:326–338.
- Eyerich K & Eyerich S. J Eur Acad 2017; 32:692–703.
- Raphael I et al. Cytokine 2015; 74:5–17.
- Schwartz DM et al. Nat Rev Rheumatol 2016; 12(1):25–36.
- Murray PJ. J Immunol 2007; 178(5):2623–2629.
- Annunziato F et al. J Allergy Clin Immunol 2014; 136(3):626–635.
- Schleimer R. Annu Rev Pathol 2017; 12:331–357.
- Nakayama T et al. Annu Rev Immunol 2017; 35:53–84.
- Coates LC et al. Semin Arthritis Rheum 2016; 46:291–304.
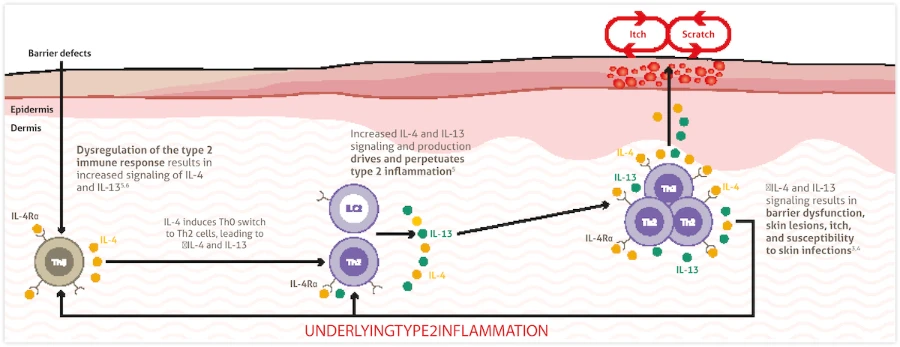
In Atopic Dermatitis, Type 2 Inflammation is always present throughout the body,even in nonlesional or normal-looking skin1–3
- Dysregulation of the type 2 immune response and increased IL-4 and IL-13 signaling drives and perpetuates type 2 inflammation, which contributes to clinical disease features in atopic dermatitis and other type 2 inflammatory diseases3-5
-
IL-4 and IL-13 are produced by various cell types such as Th2 and ILC2 cells in response to increased signaling through the IL-4Rα present on those cells6-8
- Leung DYM et al. J Clin Invest 2004; 113:651–657.
- Suárez-Fariñas M et al. J Allergy Clin Immunol 2011; 127:954–964.
- Gittler JK et al. J Allergy Clin Immunol 2012; 130:1344–1354.
- Biedermann T et al. Front Immunol 2015; 6:353.
- Gandhi NA et al. Nat Rev Drug Discov 2016; 15:35–50.
- Artis D & Spits H. Nature 2015; 517:293–301.
- McLeod JJA et al. Cytokine 2015; 75(1):57–61.
- Guttman-Yassky E et al. J Allergy Clin Immunol 2019; 143:155–172.
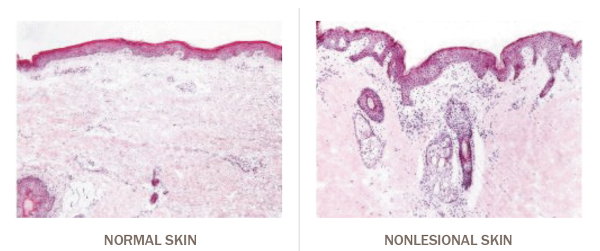
Nonlesional skin is not normal skin
In atopic dermatitis, subclinical inflammation is also present in nonlesional skin1–3
- Leung DYM et al. J Clin Invest 2004; 113:651–657.
- Suárez-Fariñas M et al. J Allergy Clin Immunol 2011; 127:954–964.
- Gittler JK et al. J Allergy Clin Immunol 2012; 130:1344–1354.
- Biedermann T et al. Front Immunol 2015; 6:353.
- Gandhi NA et al. Nat Rev Drug Discov 2016; 15:35–50.
- Artis D & Spits H. Nature 2015; 517:293–301.
- McLeod JJA et al. Cytokine 2015; 75(1):57–61.
- Guttman-Yassky E et al. J Allergy Clin Immunol 2019; 143:155–172.