Proven Efficacy of Dupixent® in COPD

COPD is a progressive lung disease characterized by airflow limitation. Exacerbations, both moderate and severe, can lead to a decline in lung function, particularly FEV-1 (Forced Expiratory Volume in 1
second) 1,2.

GOLD Group E patients, according to the GOLD (Global Initiative for Chronic Obstructive Lung Disease) classification, are those with a high risk of exacerbations and more symptoms, often experiencing a faster decline in lung function and quality of life 3.

FEV-1, or Forced Expiratory Volume in 1 second, is a key measurement in lung function testing. It represents the volume of air a person can forcefully exhale in the first second after a deep breath.
Lung function decline (FEV-1) in COPD patients increases mortality risk, whereas improvement resets the patient’s baseline and lowers exacerbation risk.

BOREAS and NOTUS outcomes are breakthrough for COPD patients, showing statistically significant and clinically meaningful improvements in moderate and severe exacerbation reduction, lung function over 52 weeks4,5.
These 2 trials have been conducted on approx. 2000 patients and its endpoints were designed according to the GOLD standards4,5.
Dupixent's significantly proven reduction of Moderate and Severe exacerbations6:
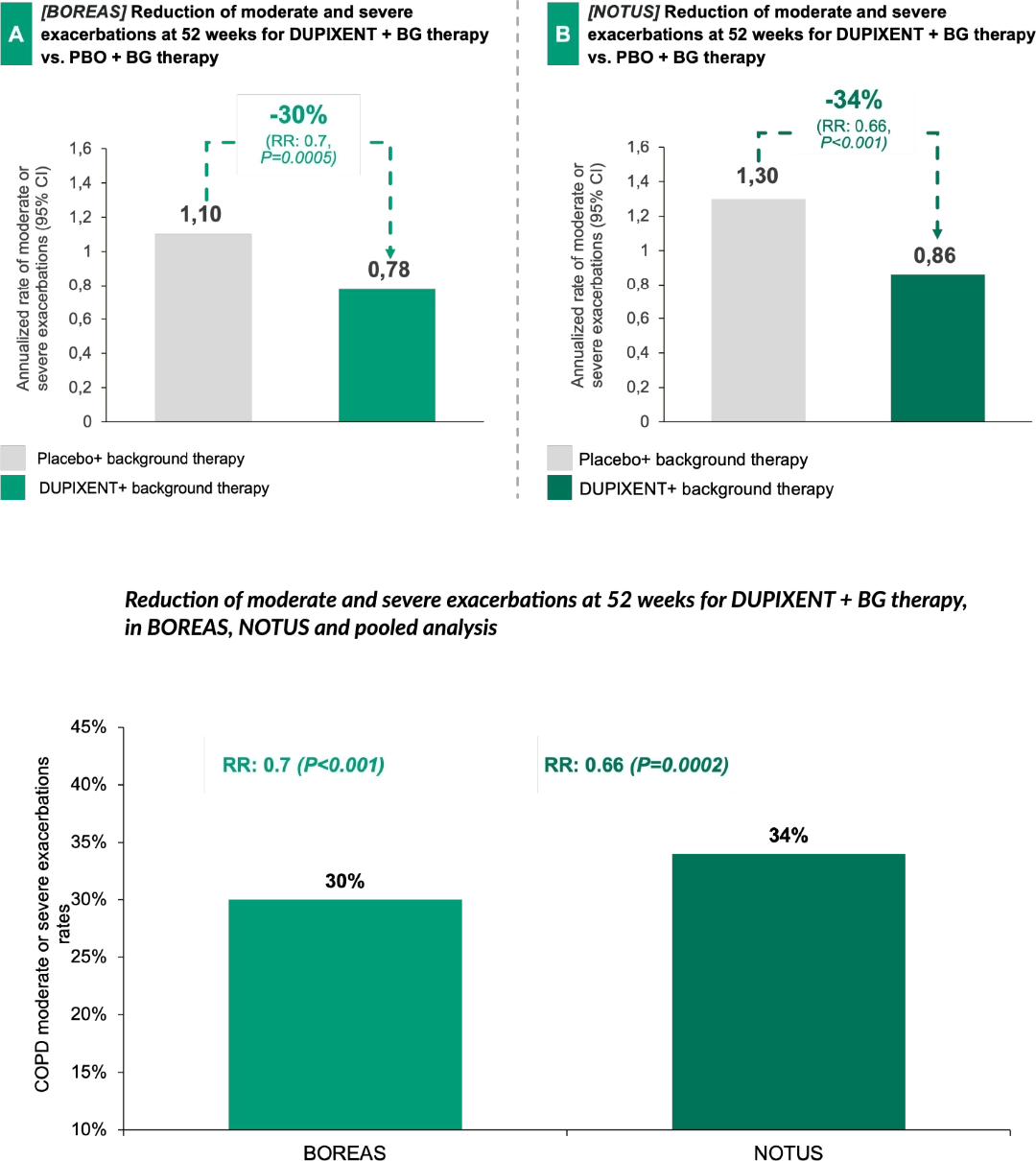

In both BOREAS and NOTUS, Dupixent + background therapy has demonstrated statistically significant reductions in moderate and severe exacerbation rate up to 34%, as shown in the NOTUS trial, vs. placebo + background therapy.
Dupixent's statistically significant FEV-1 improvement6:
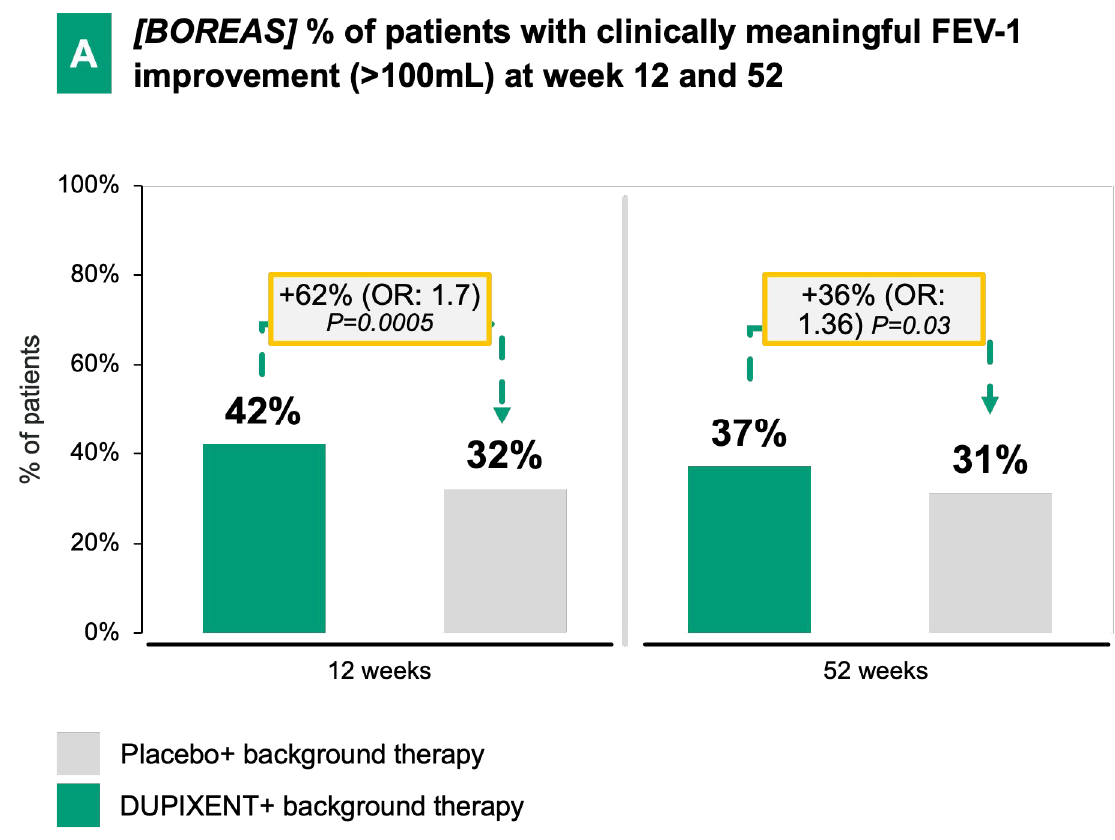
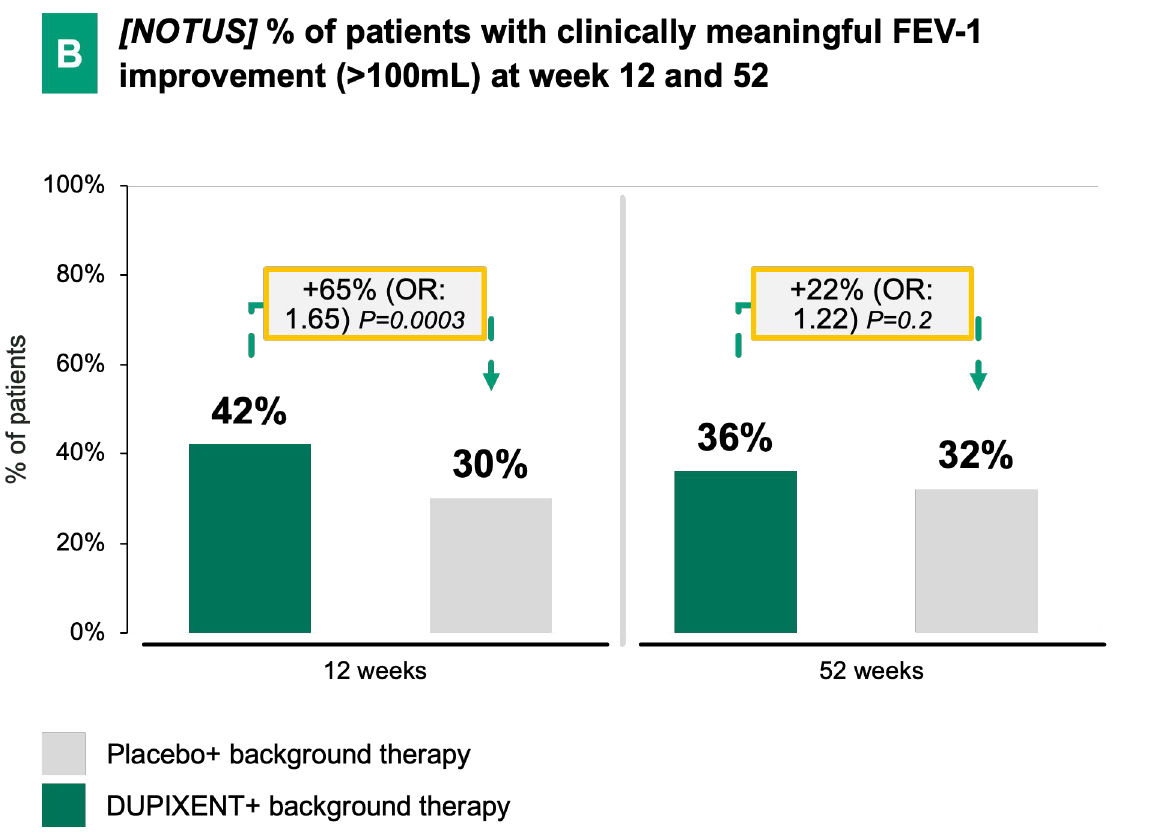
In both BOREAS and NOTUS, Dupixent demonstrated early and significant FEV-1 improvement vs. placebo + background therapy over 52 weeks.
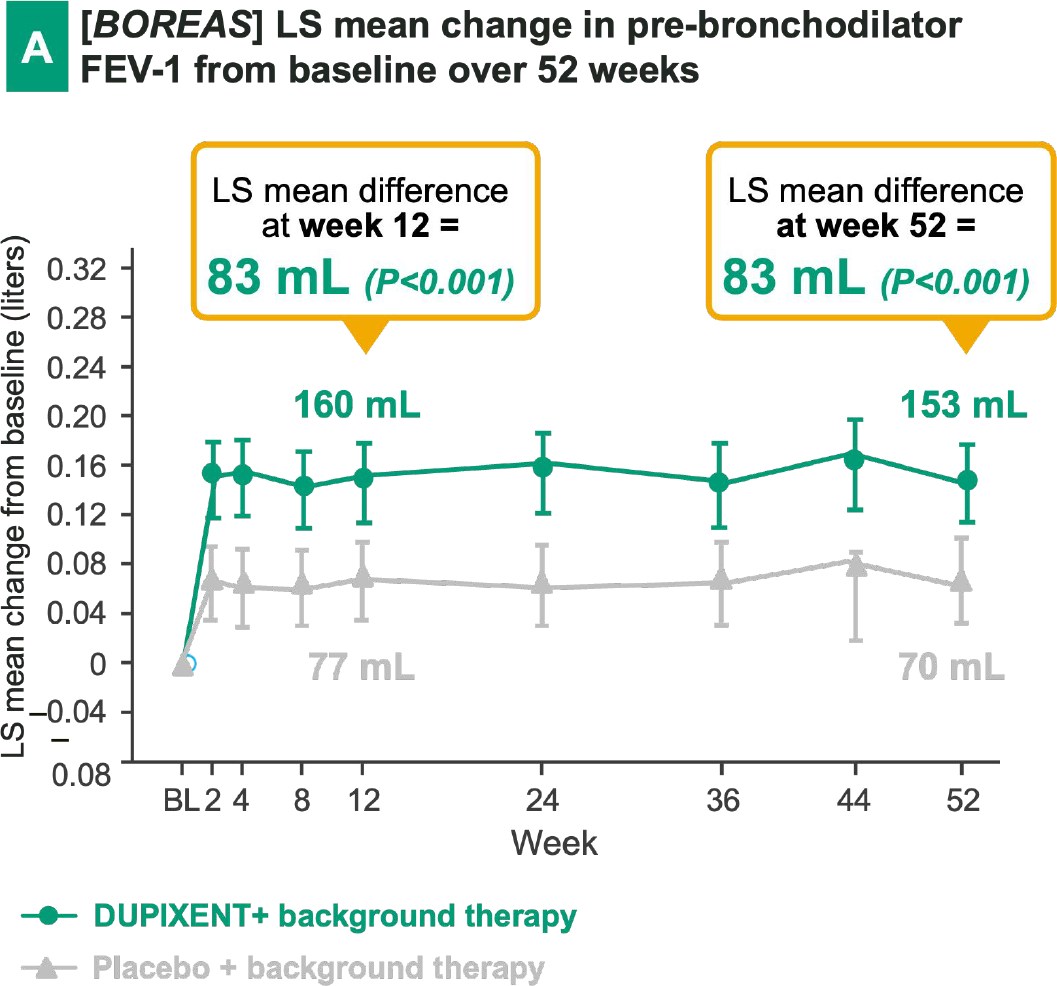
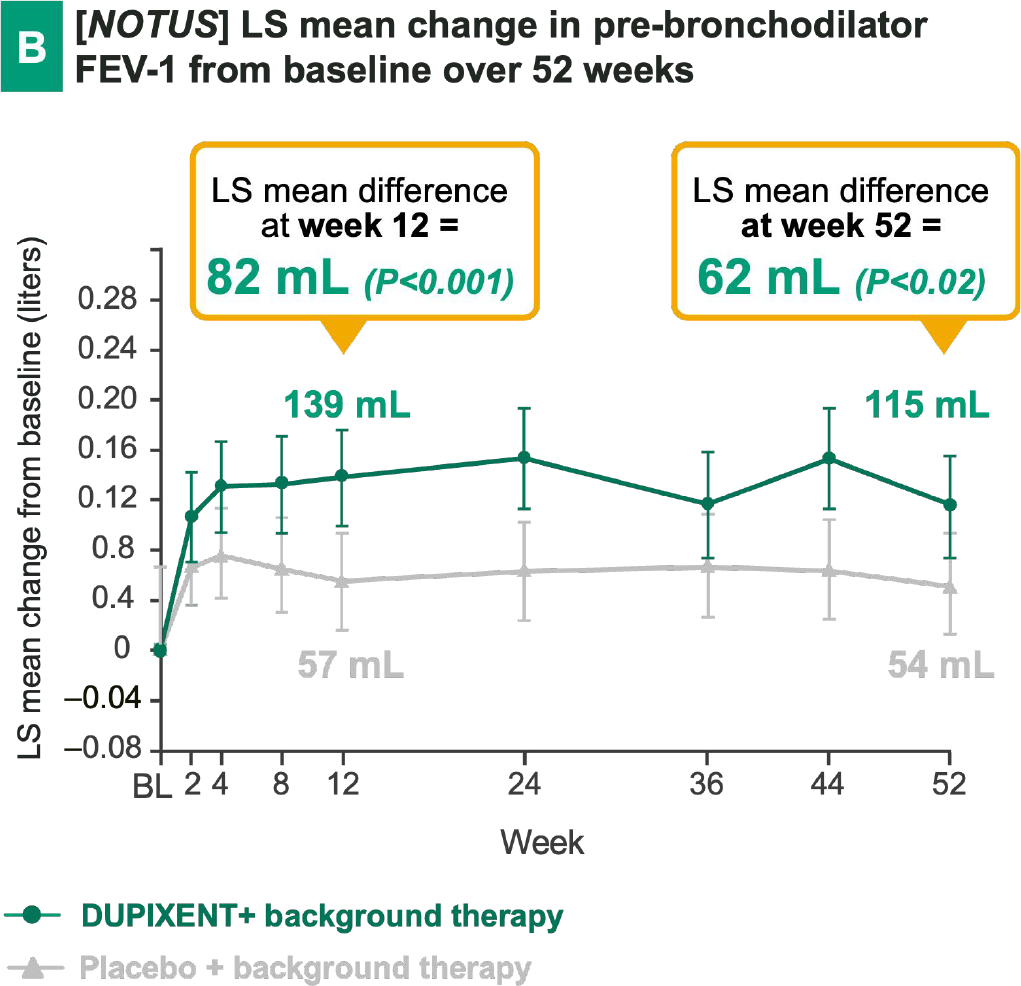
DUPIXENT + BG therapy has demonstrated a notable improvement in lung function vs. placebo + BG therapy, in particular has shown:

Early onset
(e.g., measurable effect already after 2 weeks in BOREAS)

Sustained effect
over 52 weeks in BOREAS

Significant FEV-1 improvement
at week 52 in both trials
A meta-analysis (52 studies; 62,385 patients) demonstrated significant correlations between mean trough FEV-1 and HRQOL in patients with stable COPD 1,2
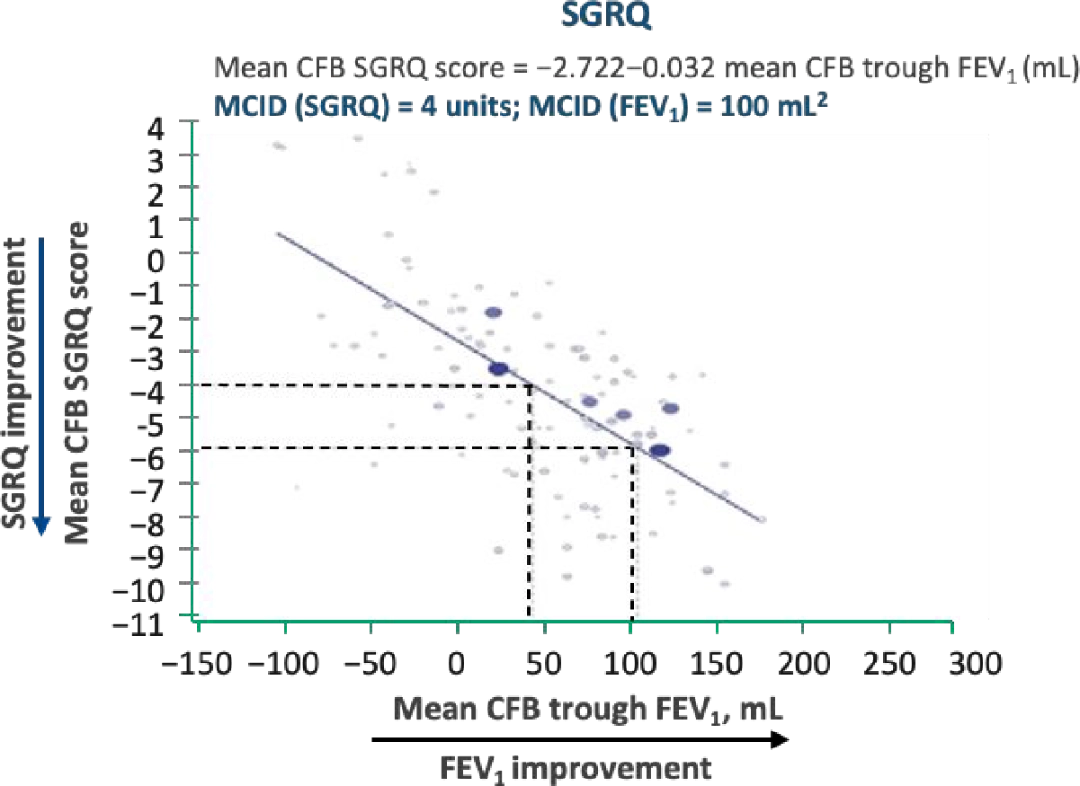
- An MCID improvement in FEV-13 corresponded to a larger than MCID improvement in measures of HRQOL (SGRQ)4 and breathlessness (TDI)4
- Analyses of follow-up data at 6 and 12 months suggest that the correlation of trough FEV-1 with HRQOL (SFRQ and TDI) strengthens with time.
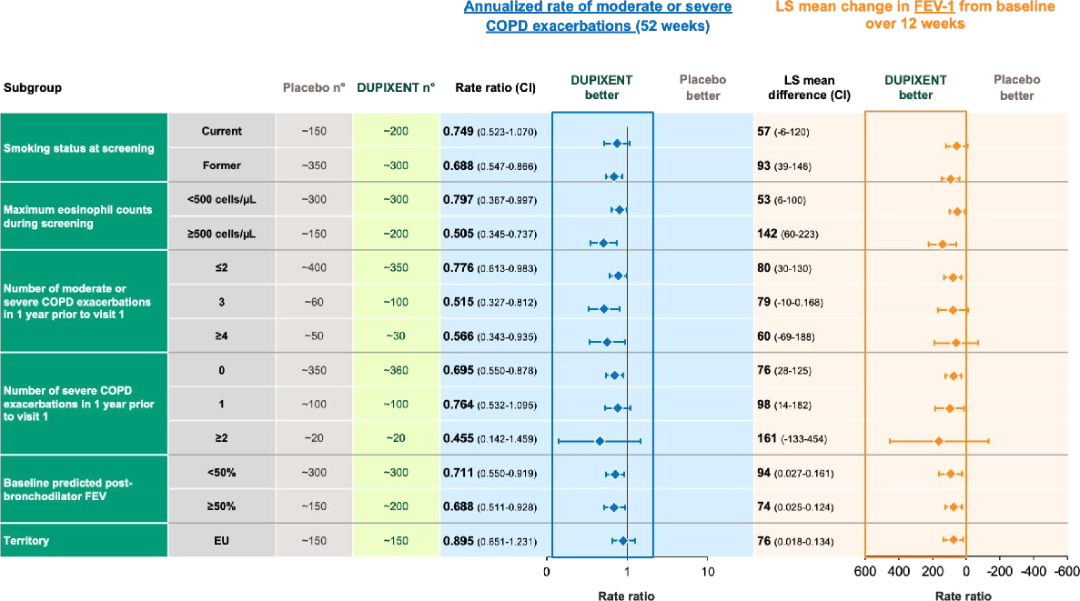
Dupixent has proven to show consistent results across subpopulations in both BOREAS and NOTUS trials6:
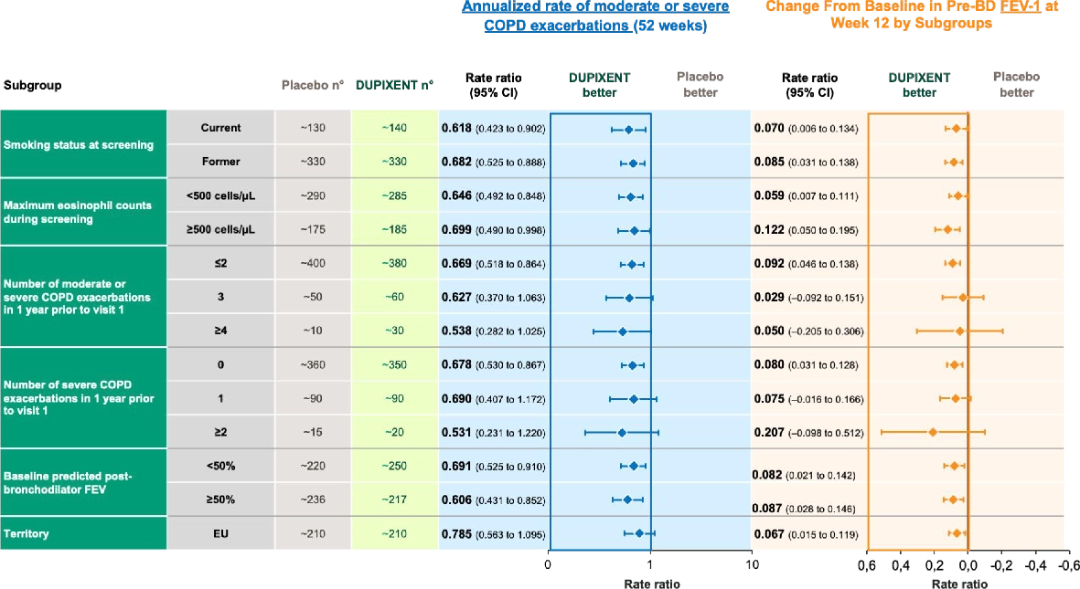
In the BOREAS trial, Dupixent demonstrated consistent clinical benefit in terms of moderate or severe exacerbation reduction and lung function (i.e., FEV-1 change vs. placebo) results across study subgroups.
In the NOTUS trial, Dupixent demonstrated consistent clinical benefit in terms of moderate or severe exacerbation reduction and lung function (i.e., FEV-1 change vs. placebo) results across study subgroups.
- Wedzicha, J. A., & Seemungal, T. A. R. (2007). COPD exacerbations: Defining their cause and prevention. The Lancet, 370(9589), 786-796. https:// doi.org/10.1016/S0140-6736(07)61382-8
- MacIntyre, N., & Huang, Y. C. (2008). Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society, 5(4), 530-535. https://doi.org/10.1513/pats.200707-088ET
- Kim, V., & Aaron, S. D. (2018). What is a COPD exacerbation? Current definitions, pitfalls, challenges, and opportunities for improvement. European Respiratory Journal, 52(5), 1801261. https://doi.org/10.1183/13993003.01261-2018
- Bhatt, et al. NJEM. 2023; 389:205-214
- Bhatt, et al. NJEM. 2024; DOI: 10.1056/NEJMoa2401304
- Simon-Kucher; Sanofi and Regeneron

