in Asthma`, author: ``, tags: `Asthma | cutting-edge-science`, publication_date: ``, interaction_type: "content" }
Airway Hyperresponsiveness
in Asthma
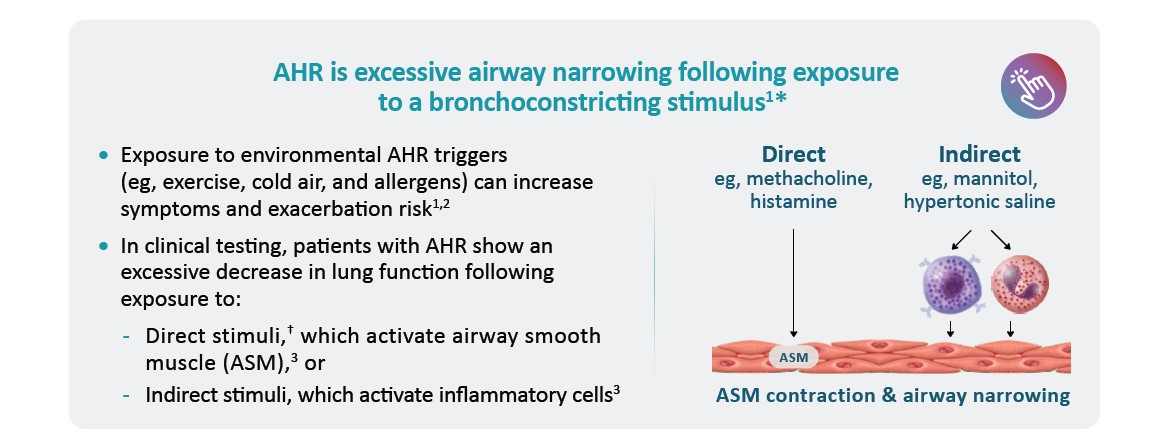










*AHR tests are not commonly performed in clinical practice, as they are not necessary for asthma diagnosis and are not readily available in primary care.7 †AHR tests done with direct stimuli, such as the methacholine challenge test, may induce adverse effects, including severe bronchospasm.8
AHR, airway hyperresponsiveness; ASM, airway smooth muscle.
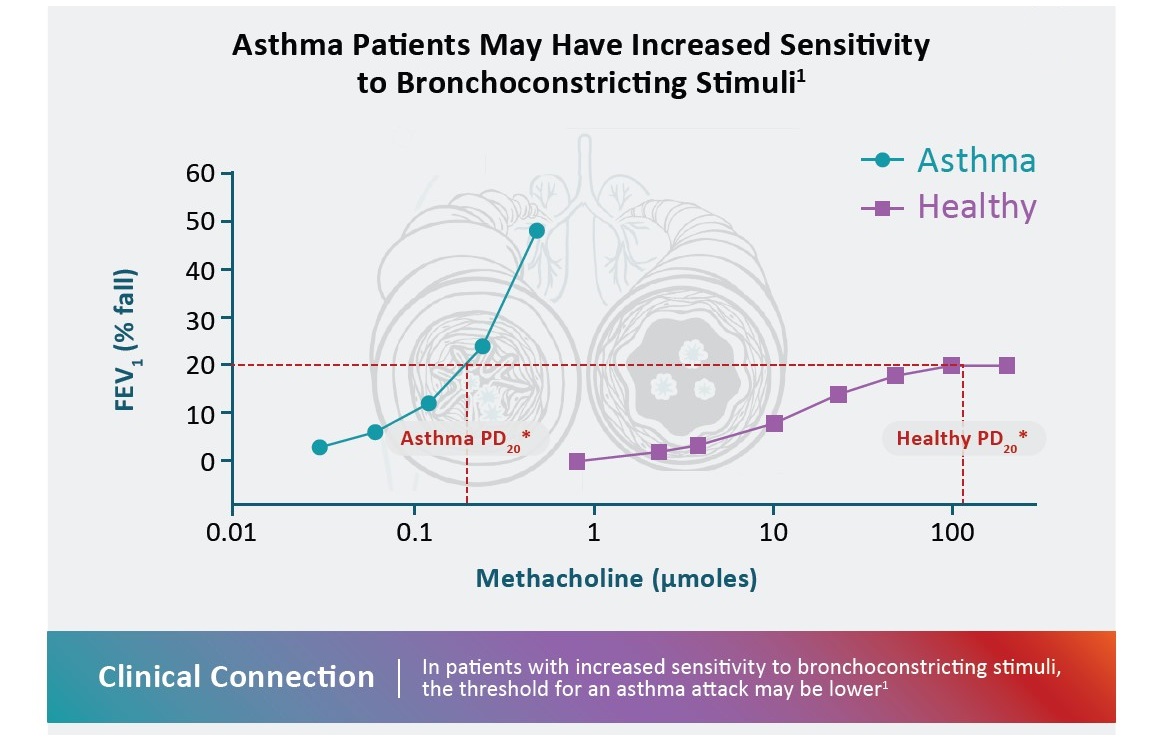
*PD20 and PC20 values are common endpoints of tests for airway hyperresponsiveness.1,3
FEV1, forced expiratory volume in 1 second; PC20, provocative concentration that causes a 20% fall in FEV1; PD20, provocative dose that causes a 20% fall in FEV1.
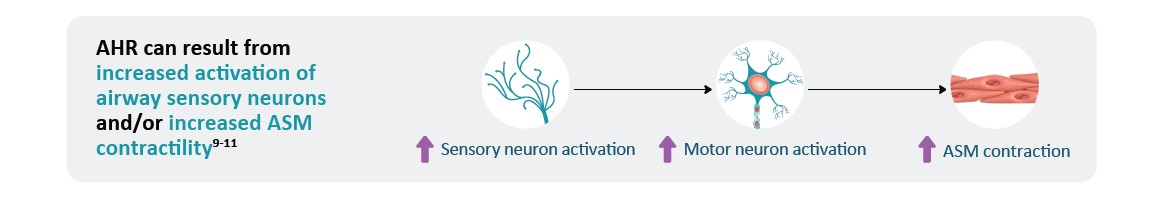
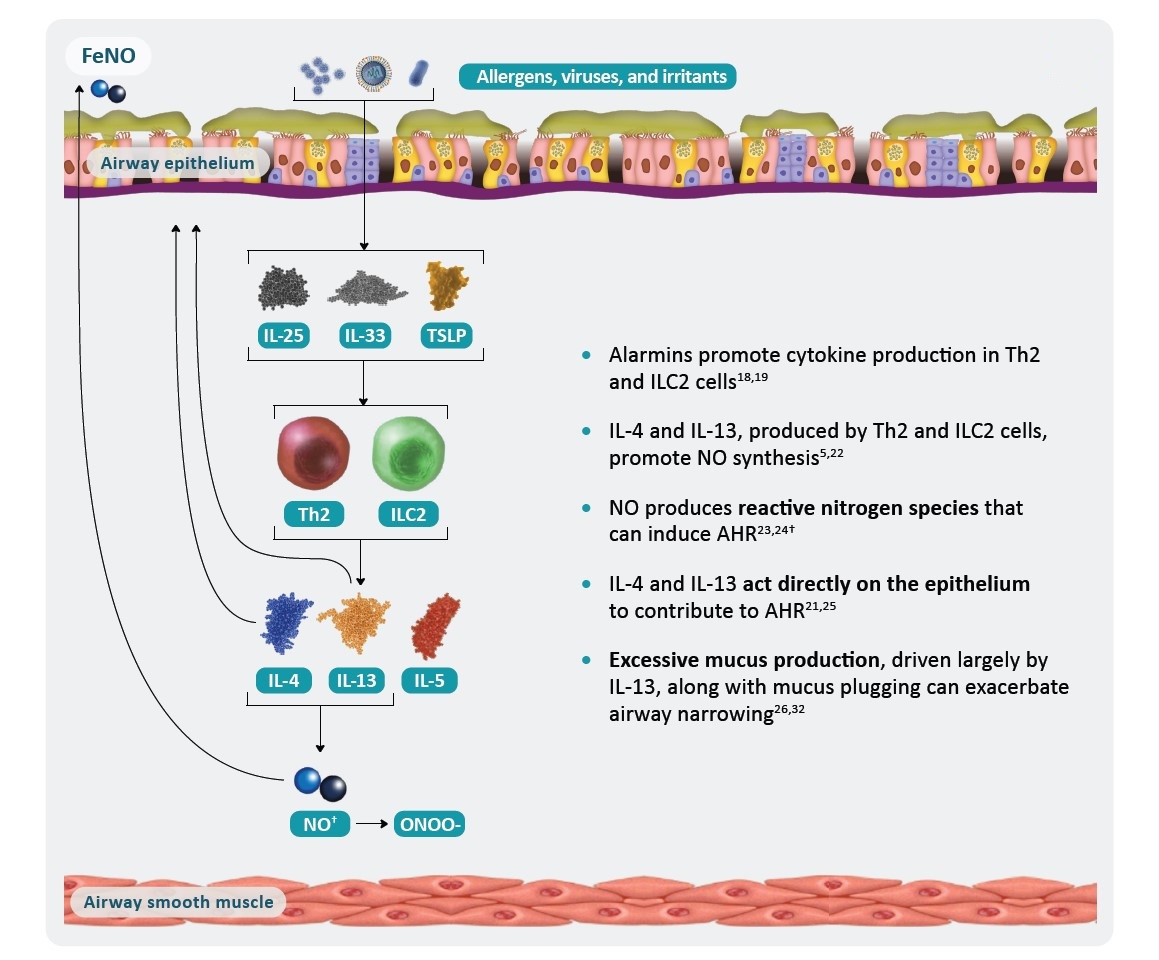
*AHR is a multifaceted process that is not yet fully understood and includes components in addition to those shown here.1 †Failure of NO to exert bronchodilatory and anti-inflammatory effects may also contribute to AHR.29
AHR, airway hyperresponsiveness; ASM, airway smooth muscle; EOS, eosinophil; IL, interleukin; ILC2, group 2 innate lymphoid cell; NO, nitric oxide; RNS, reactive nitrogen species; Th2, T helper type 2; TNF, tumor necrosis factor; TSLP, thymic stromal lymphopoietin.
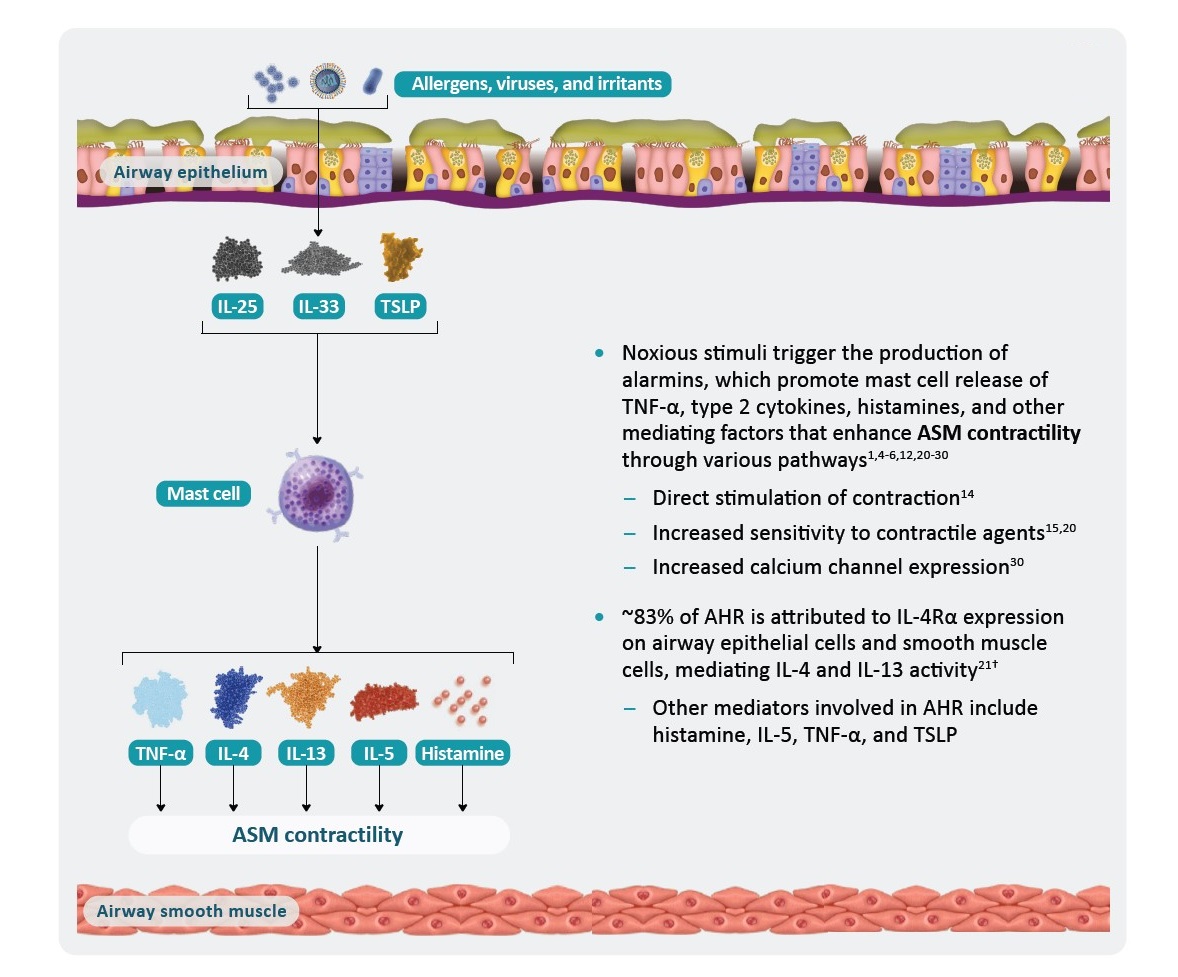
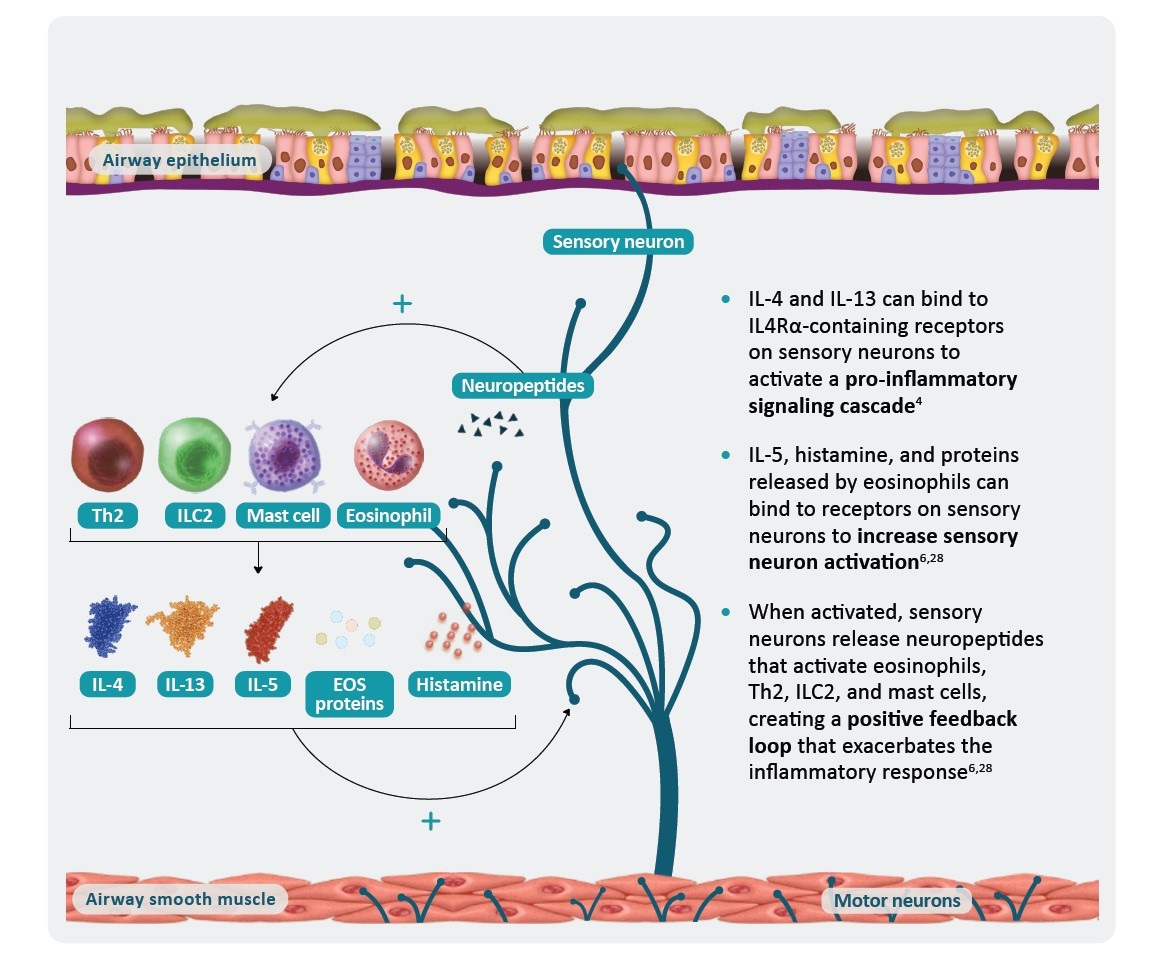
*AHR is a multifaceted process that is not yet fully understood and includes components in addition to those shown here.1
†In a murine allergic airway disease mouse model for human asthma.21
AHR, airway hyperresponsiveness; ASM, airway smooth muscle; EOS, eosinophil; IL, interleukin; R, receptor; TNF, tumor necrosis factor; TSLP, thymic stromal lymphopoietin.
*AHR is a multifaceted process that is not yet fully understood and includes components in addition to those shown here.1
†Failure of NO to exert bronchodilatory and anti-inflammatory effects may also contribute to AHR.29
AHR, airway hyperresponsiveness; ASM, airway smooth muscle; FeNO, fractional exhaled nitric oxide; IL, interleukin; ILC2, group 2 innate lymphoid cell; NO, nitric oxide; ONOO-, peroxynitrite; Th2, T helper type 2; TSLP, thymic stromal lymphopoietin.
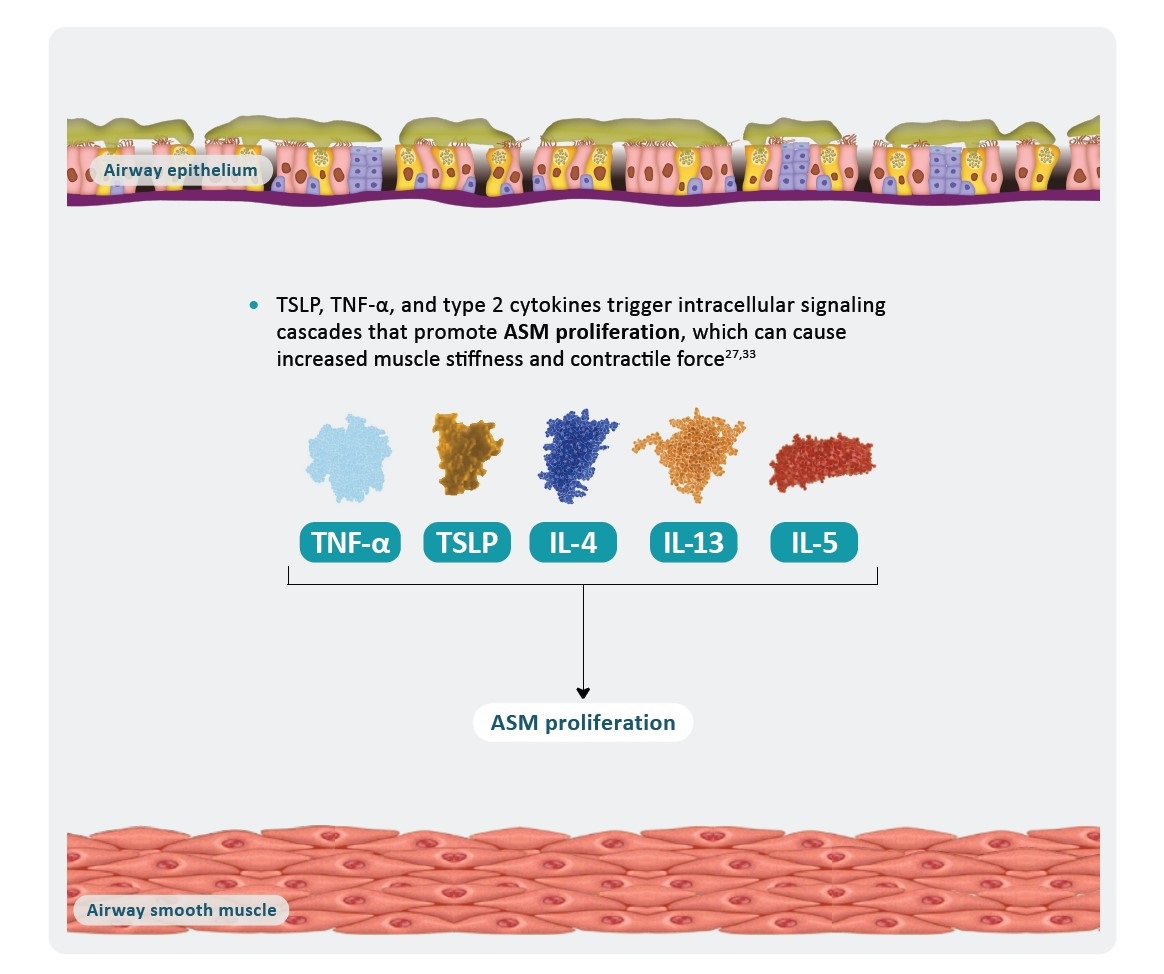
*AHR is a multifaceted process that is not yet fully understood and includes components in addition to those shown here.1
AHR, airway hyperresponsiveness; ASM, airway smooth muscle; IL, interleukin; TNF, tumor necrosis factor; TSLP, thymic stromal lymphopoietin.
*AHR is a multifaceted process that is not yet fully understood and includes components in addition to those shown here.1
AHR, airway hyperresponsiveness; ASM, airway smooth muscle; EOS, eosinophil; IL, interleukin; ILC2, group 2 innate lymphoid cell; R, receptor; Th2, T helper type 2.
1. Chapman DG, Irvin CG. Clin Exp Allergy. 2015;45(4):706-719.
2. Cockcroft DW, Davis BE. J Allergy Clin Immunol.
2006;118(3):551-561.
3. Brannan JD, Lougheed MD. Front Physiol. 2012;3:460.
4. Crosson T, et al. bioRxiv. Preprint posted online January 27, 2023. doi:10.1101/ 2023.01.26.525731
5. Gandhi NA, et al. Nat Rev Drug Discov. 2016;15(1):35-50.
6. Kabata H, Artis D. J Clin Invest. 2019;129(4):1475-1482.
7. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Updated July 2023. Accessed August 14, 2023. https://ginasthma.org/gina-reports/
8. Coates AL, et al. Eur Respir J. 2017;49(5):1601526.
9. Pincus AB, et al. Neurosci Lett. 2021;751:135795.
10. Lauzon AM, Martin JG. F1000Res. 2016;5:F1000 faculty Rev- 306.
11. Kistemaker LEM, Prakash YS. Physiology (Bethesda).
2019;34(4):283-298.
12. Bradding P. Eur Respir J. 2007;29(5):827-830.
13. Banafea GH, et al. Bioengineered. 2022;13(3):7049-7064.
14. Yamauchi K, Ogasawara M. Int J Mol Sci. 2019;20(7):1733.
15. Amrani Y, et al. Respir Res. 2000;1(1):49-53.
16. Manson ML, et al. J Allergy Clin Immunol. 2020;145(3):808-817.e2.
17. Altman MC, et al. J Clin Invest. 2019;129(11):4979-4991.
18. Hong H, et al. Allergy. 2020;75(11):2794-2804.
19. Whetstone CE, et al. Cells. 2022;11(7):1105.
20. Rizzo CA, et al. J Allergy Clin Immunol. 2002;109(3):404-409.
21. McKnight CG, et al. Mucosal Immunol. 2020;13(2):283-292.
22. Alving K, Malinovschi A. Basic aspects of exhaled nitric
oxide. In: Horvath, de Jongste JC, eds. Exhaled Biomarkers. European Respiratory Society; 2010:1-33. European Respiratory Monograph No. 49.
23. Prado CM, et al. ISRN Allergy. 2011;2011:832560.
24. Sugiura H, Ichinose M. Antioxid Redox Signal. 2008;10(4):785-797.
25. Perkins C, et al. J Allergy Clin Immunol. 2006;118(2):410-419.
26. Kuperman DA, et al. Nat Med. 2002;8(8):885-889.
27. Prakash YS. Am J Physiol Lung Cell Mol Physiol.
2013;305(12):L912-L933.
28. Talbot S, et al. Annu Rev Immunol. 2016;34:421-447.
29. Meurs H, et al. Eur Respir J. 2008;32(2):487-502.
30. Ding S, et al. Clin Exp Pharmacol Physiol. 2019;46(1):56-64.
31. Yeh SY, Schwartzstein R. Asthma, Health and Society. 2009;19-42.
32. Aegerter H, Lambrecht BN. Annu Rev Pathol. 2023;18:387-409.
33. Doeing DC, Solway J. J Appl Physiol (1985). 2013;114(7):834-843.