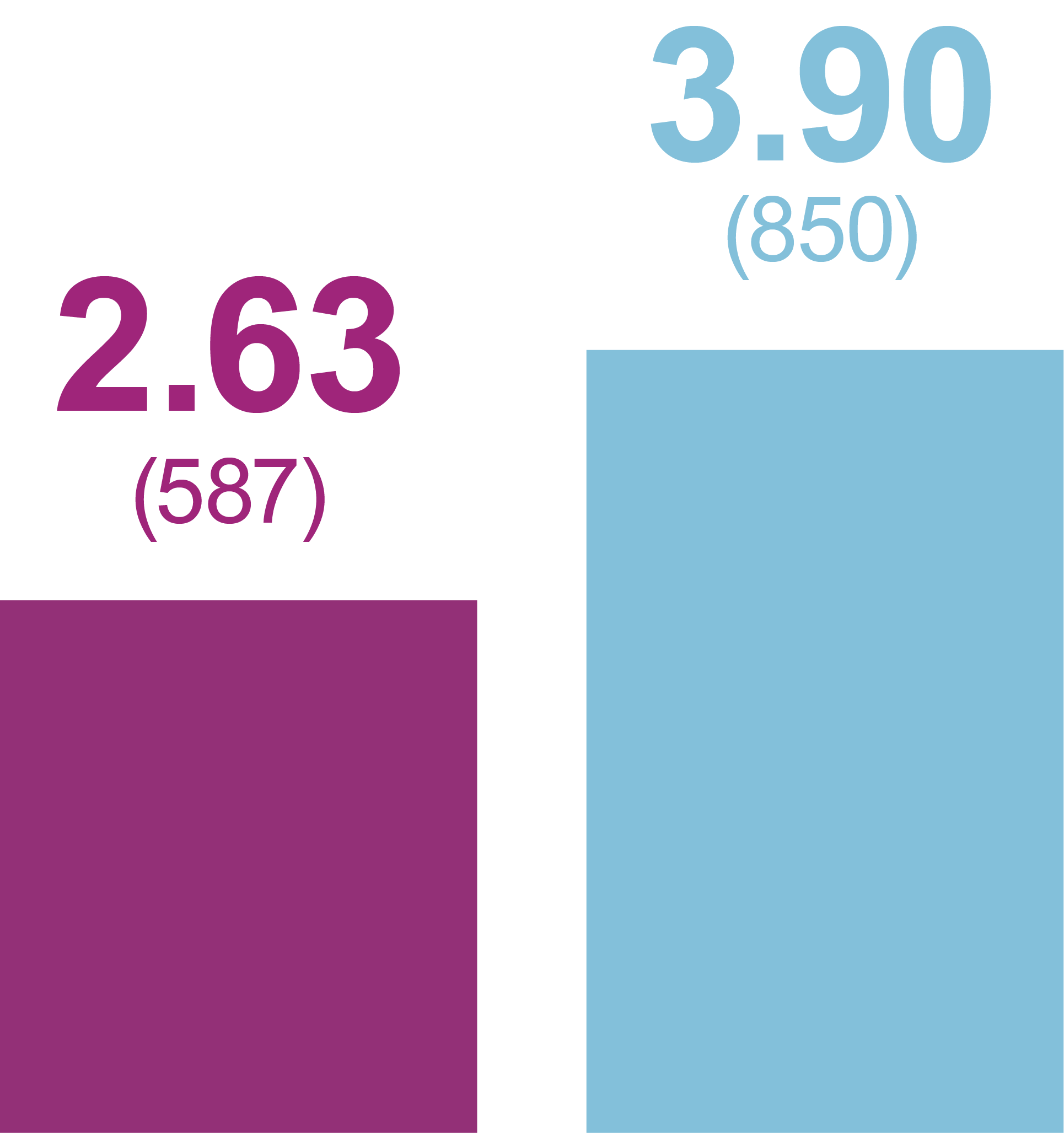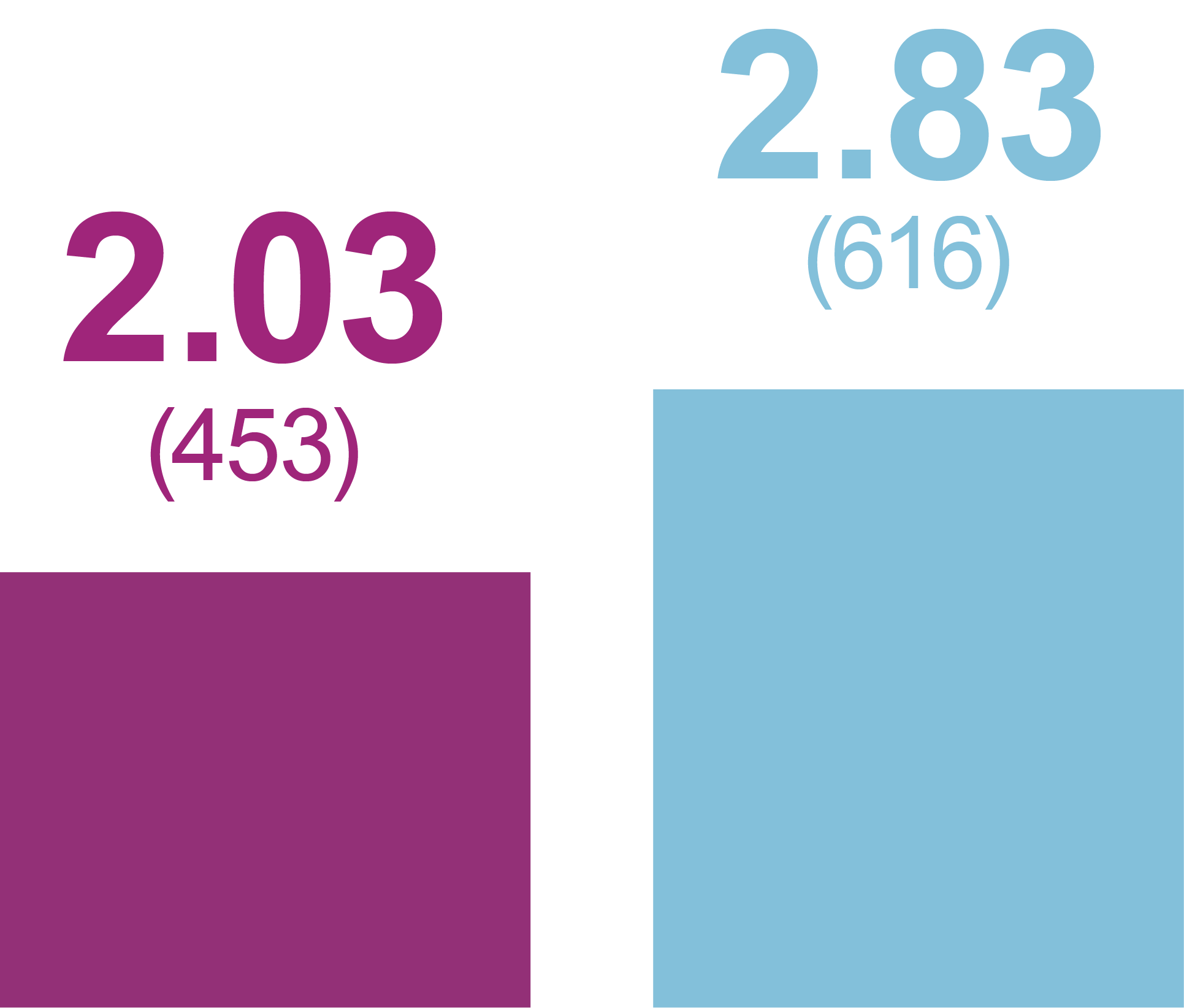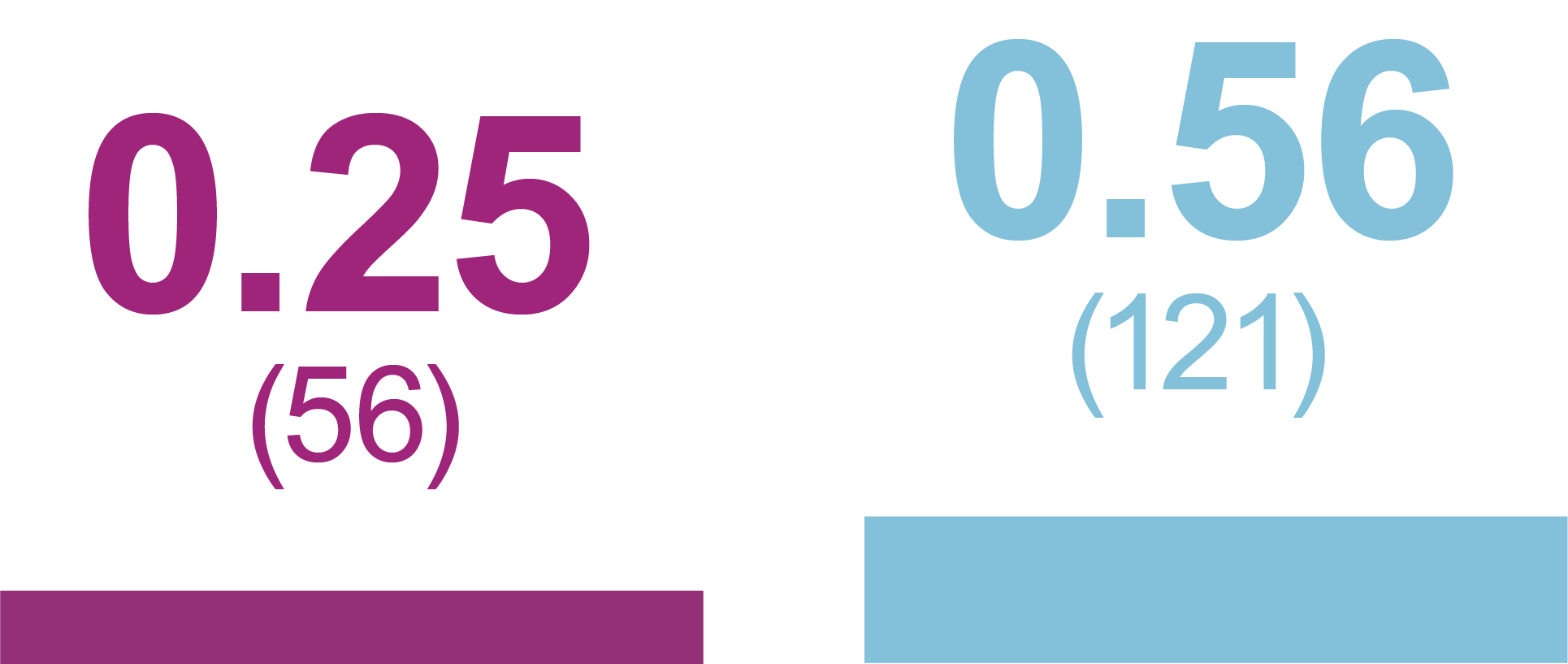Advancing therapy in suboptimally controlled basal insulin-treated type 2 diabetes: Clinical outcomes with iGlarLixi versus premix BIAsp 30 in the SoliMix randomized controlled trial.
Advancing therapy in Suboptimallycontrolled Basal insulin-treated Type 2 Diabetes: Clinical outcomes with iGlarLixi versus premix BIAsp 30 in the SoliMix randomized controlled trial
Introduction
- For people with T2D on basal insulin who fail to reach their recommended glycemic targets, options for treatment advancement include:
- Basal insulin + progressive addition of rapid-acting insulin
- Premix insulin
- Basal insulin + GLP-1 RA
- Fixed-ratio combination (FRC) of basal insulin + GLP-1 RA
-
All these treatment options have been shown to improve glycemic control when used to advance therapy from basal insulin, but are associated with specific side-effects:
- GLP-1 RA therapy can be associated with gastrointestinal AEs and resultant adherence issues
- Basal + rapid-acting insulin and premix insulin regimens can increase the risk of hypoglycemia and weight gain, require multiple daily injections and frequent glucose monitoring
- Globally, premix insulins are widely used to advance insulin therapy from basal insulin
- iGlarLixi is a once-daily titratable FRC of basal insulin glargine 100 U/mL (iGlar) and the GLP-1 RA, lixisenatide (Lixi), which offers an alternative option to premix insulin for treatment advancement
Objective
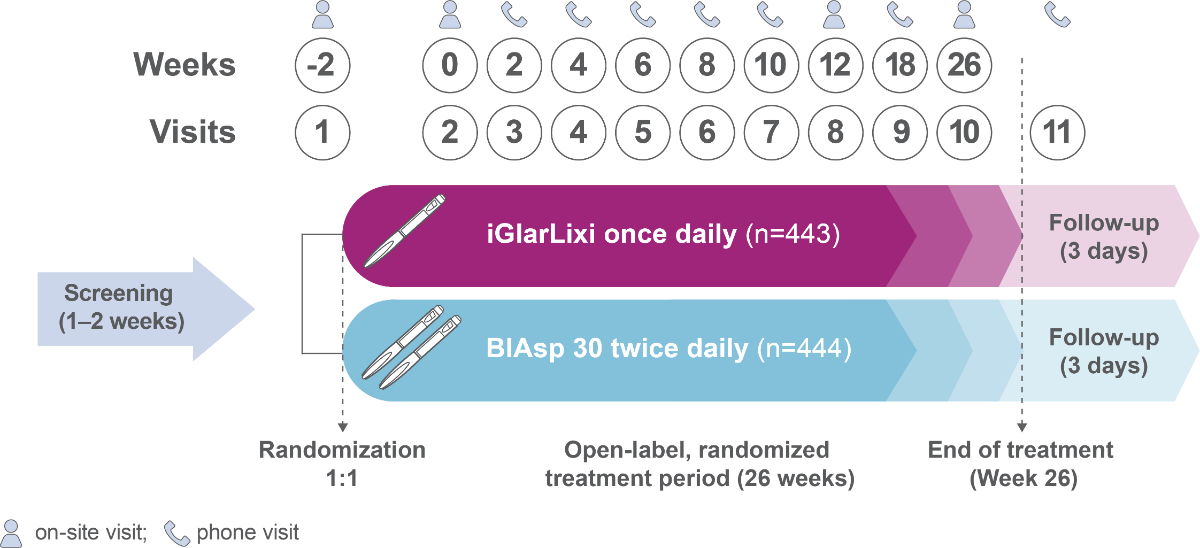
SoliMix is the first RCT to directly compare the efficacy and safety of an FRC of basal insulin and GLP-1 RA, iGlarLixi, with premix insulin analog, biphasic insulin aspart 30 (BIAsp 30), in people with T2D advancing from basal insulin plus one or two OADs
Methods
Population
- Adults (≥18 years)
- Suboptimally controlled by basal insulin combined with metformin ± SGLT2i
- HbA1c of ≥7.5 % and ≤10.0 % (≥58.5–≤85.8 mmol/mol)
Primary efficacy endpoints
| Mean ± SD age, years |
Sex, |
Mean ± SD BMI, kg/m2 |
Mean ± SD duration of T2D, years |
Mean ± SD HbA1c |
||
| % | mmol/mol | |||||
| iGlarLixi (n=443) |
59.8 ±10.3 |
49.4% | 29.7 ±4.7 |
13.0 ±7.1 |
8.6 ±0.7 |
71 ±7 |
| BIAsp 30 (n=444) |
59.8 ±10.0 |
50.9% | 30.0 ±5.1 |
13.0 ±7.4 |
8.6 ±0.7 |
70 ±7 |
Further details on baseline characteristics have been pr eviously published1
Key results: Efficacy
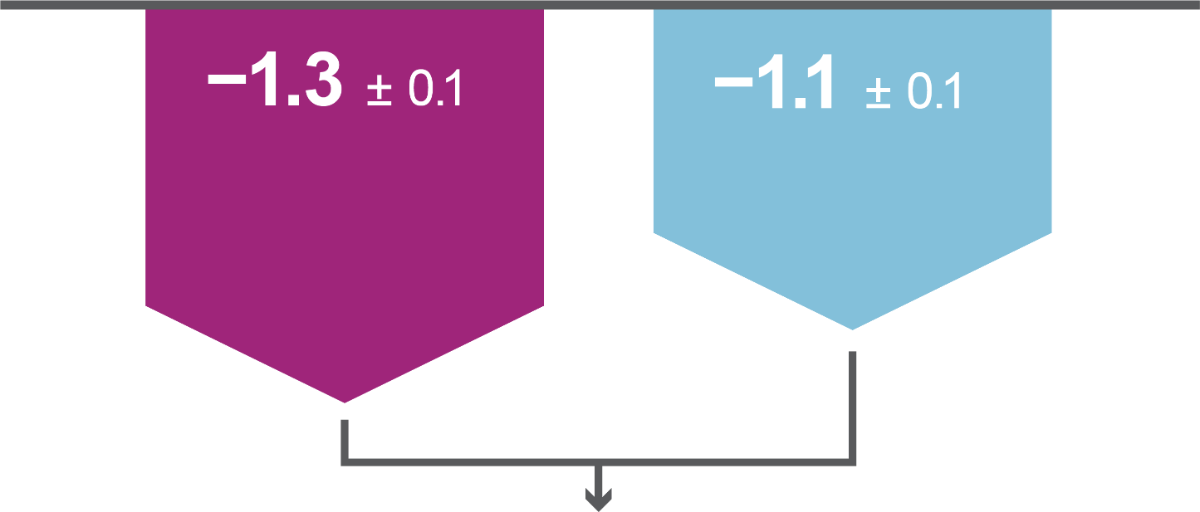
Both primary efficacy endpoints were met: iGlarLixi, demonstrated non-inferiority in HbA1c reduction and superiority in bodyweight change from baseline to Week 26 versus BIAsp 30 (ITT population). Secondary analyses showed iGlarLixi was superior to BIAsp 30 in HbA1c reduction.
iGlarLixi(n=443)
LS mean ± SE change in HbA1c from baseline to Week 26, %
VS
BIAsp 30 (n=444)
LS mean ± SE change in bodyweight from baseline to Week 26, kg
Secondary efficacy endpoints
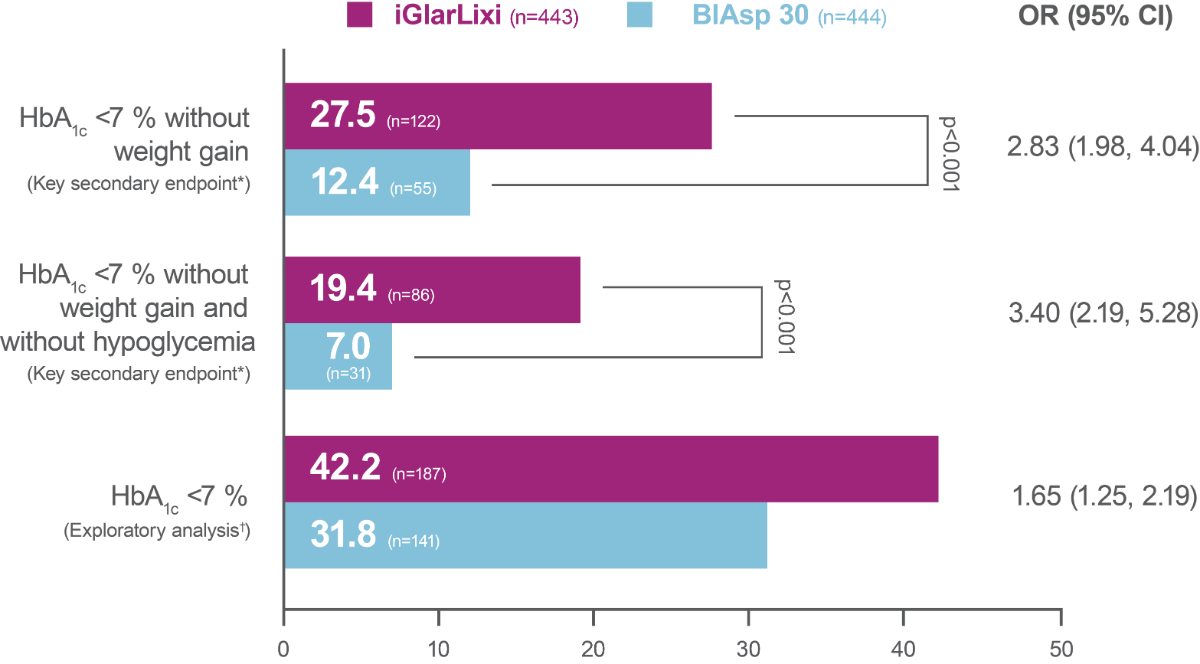
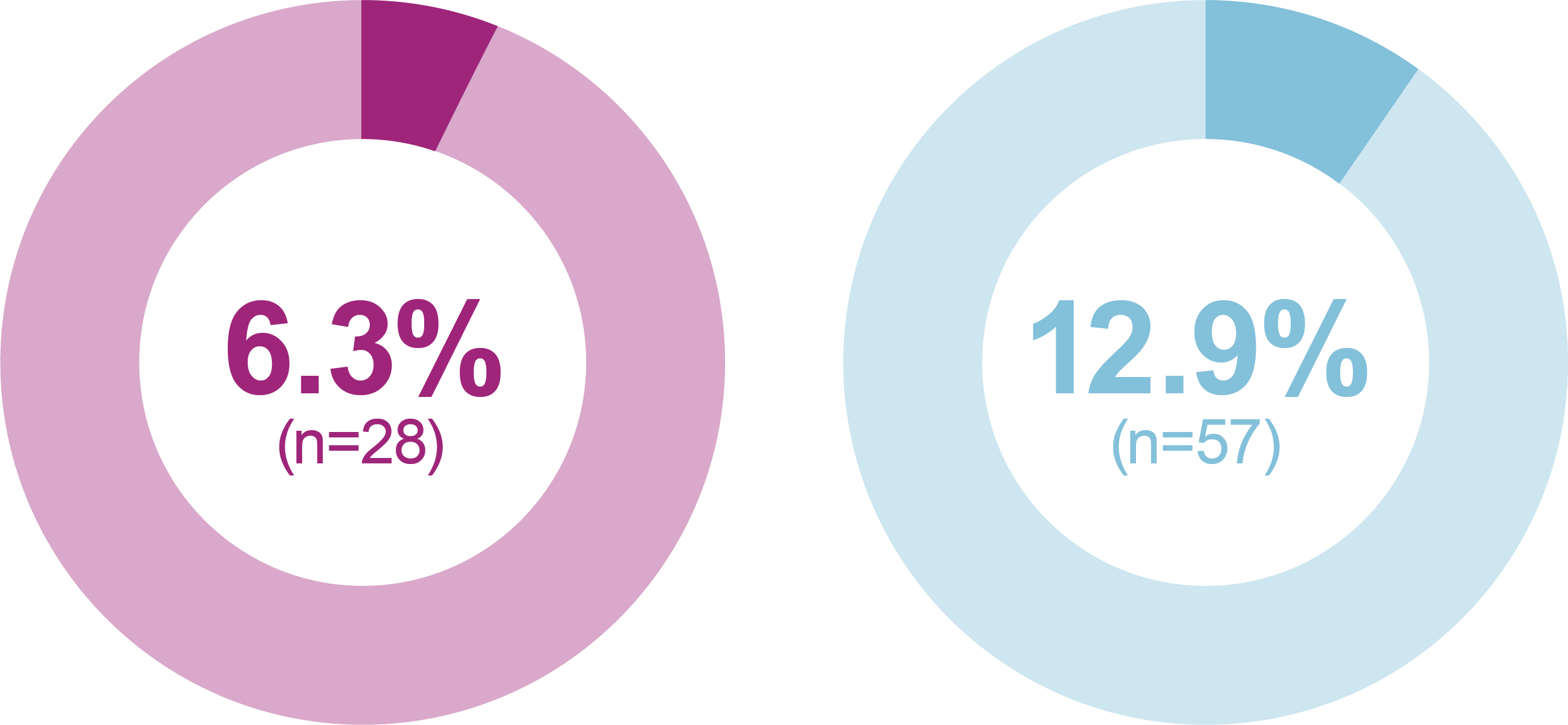
Significantly greater proportion of participants reached HbA1c <7 % (<53.0 mmol/mol) without weight gain at Week 26 or HbA1c <7 % (<53.0 mmol/mol) without weight gain at Week 26 and without hypoglycemia (<70 mg/dL [<3.9 mmol/L]) during the treatment period in the iGlarLixi versus BIAsp 30 group (key secondary endpoints*; ITT population)
The proportion of participants reaching HbA1c <7 % was greater with iGlarLixi versus BIAsp 30 (exploratory analysis†; ITT population)
Percentage of participants reaching target
* hierarchical analysis adjusted for multiplicity; †not included in the multiple testing process
Key results: safety
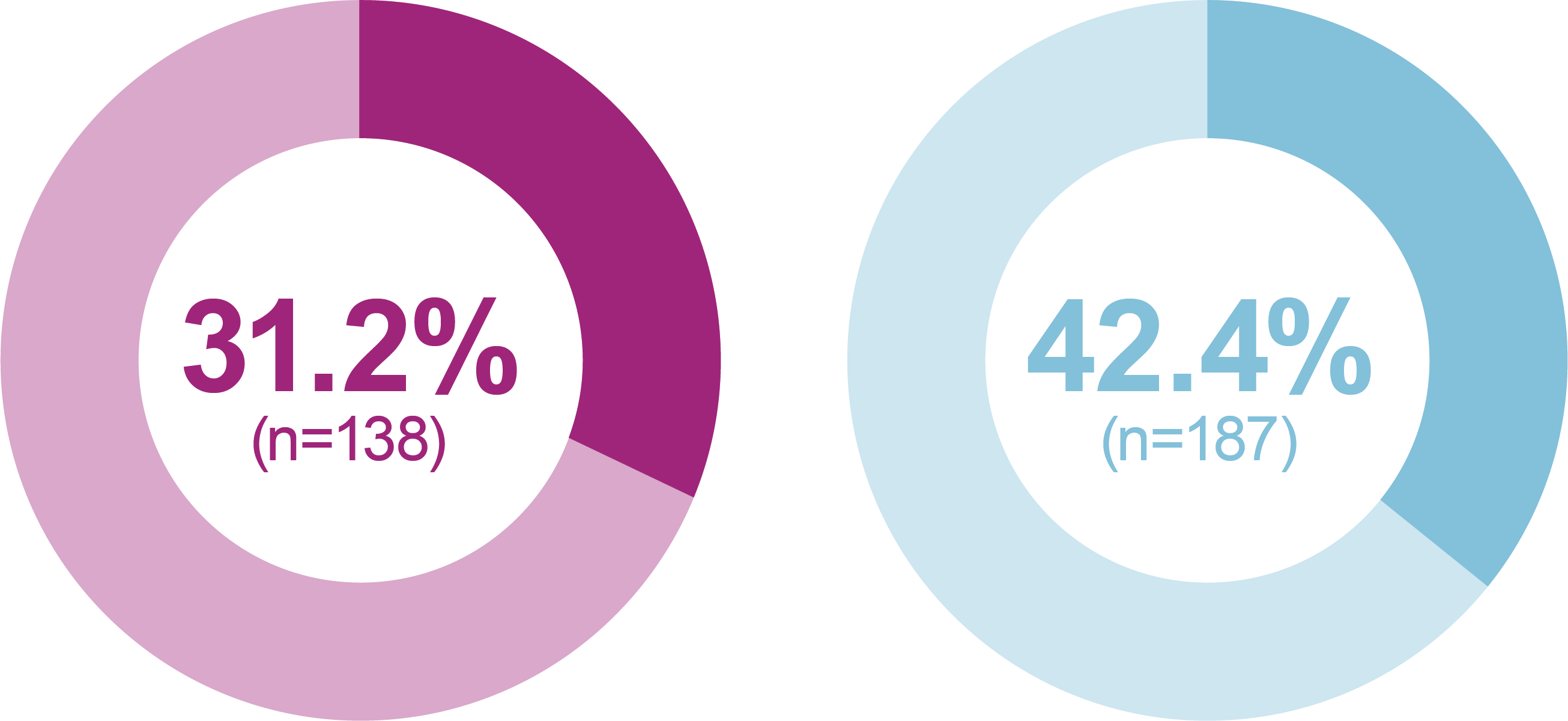
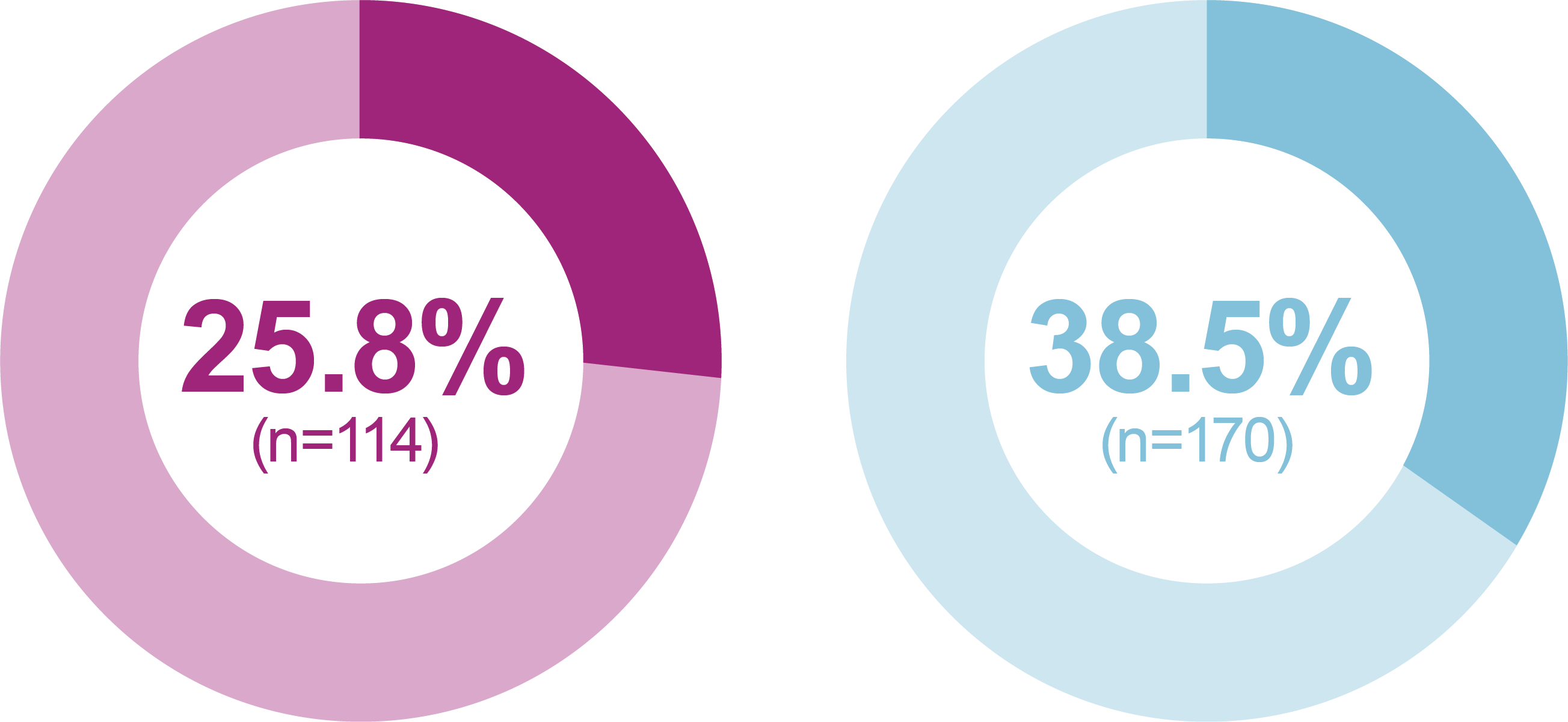
- iGlarLixi demonstrated lower incidence and rates of ADA Level 1 and Level 2 hypoglycemia compared with BIAsp 30 (safety population)
- iGlarLixi also demonstrated lower incidence and rates of ADA Level 2 hypoglycemia compared with BIAsp 30 between bedtime and waking, or between midnight and 6am
- ADA Level 3 hypoglycemia (severe hypoglycemia; any event with severe cognitive impairment requiring assistance for recovery) was low with one event in the iGlarLixi group and two events in the BIAsp 30 group
Incidence of anytime hypoglycemia, % (n)
Any hypoglycemic event
Level 1 hypoglycemia
(ADA definition: <70 mg/dL
[<3.9 mmol/L] and ≥54 mg/dL
[≥3.0 mmol/L])
Level 2 hypoglycemia
(ADA definition: <54 mg/dL [<3.0 mmol/L])
Relative odds reduction with iGlarLixi versus BIAsp 30
- -38%
- OR (95% CI): 0.62 (0.47, 0.81)
- -45%
- OR (95% CI): 0.55 (0.42, 0.74)
- -55%
- OR (95% CI): 0.45 (0.28, 0.73)
Rates of anytime Hypoglycemia, PPY (Events)
Any hypoglycemic event
Level 1 hypoglycemia
(ADA definition: <70 mg/dL [<3.9 mmol/L] and ≥54 mg/dL [≥3.0 mmol/L])
Level 2 hypoglycemia
(ADA definition: <54 mg/dL
[<3.0 mmol/L])
Relative rate reduction with iGlarLixi versus BIAsp 30
- -33%
- RR (95% CI): 0.67 (0.49, 0.91)
- -29%
- RR (95% CI): 0.71 (0.52, 0.99)
- -60%
- RR (95% CI): 0.40 (0.23, 0.71)
The most commonly reported treatment-emergent AE in the iGlarLixi iGlarLixi group was nausea (7.7% vs 0% for BIAsp 30) while nasopharyngitis was the most commonly reported treatment-emergent AE in the BIAsp 30 group (2.7% vs 3.2% for iGlarLixi) (safety population)
| n (%) | iGlarLixi (n=442) |
BIAsp 30 (n=441) |
| Any treatment-emergent AE | 144 (32.6) | 122 (27.7) |
| Any serious AE | 12 (2.7) | 13 (2.9) |
| Any AE leading to treatment discontinuation |
4 (0.9) | 4 (0.9) |
| Any AE leading to death |
0 (0) | 2 (0.5) |
Conclusions
- Compared with twice-daily premix BIAsp 30 once-daily FRC iGlarLixi demonstrated better glycemic control and weight benefit with less hypoglycemia
- iGlarLixi is a more efficacious, simpler, and well-tolerated alternative to premix BIAsp 30 for advancing therapy in people with T2D previously suboptimally controlled with basal insulin plus OADs
ADA, American Diabetes Association; AE, adverse event; BIAsp 30, biphasic insulin aspart 30 (30% insulin aspart + 70% insulin aspart protamine); BMI, body mass index; CI, confidence interval; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated hemoglobin; iGlarLixi, a fixed-ratio combination of insulin glargine 100 U/mL and the GLP-1 RA, lixisenatide; ITT, intent-to-treat; LS, l east squares; OAD, oral antihyperglycemic drug; OR, odds ratio; PPY, per participant-year; PY, participant-year; RCT, randomized controlled trial; RR, rate ratio; SD, standard deviation; SE, standard error; SGLT2i, sodium glucose co-transporter 2 inhibitor; T2D, type 2 diabetes
- McCrimmon et al. Diabetes, Obes Metab. 2021;23(6):1221–31

.png/jcr:content/Diabetes%20Science%20(1).png)
.png)