For patients 9 months of age and older
The only yellow fever vaccine available in Canada*
YF-VAX® is indicated for active immunization for the prevention of yellow fever in persons 9 months of age or older. It is indicated for both primary and booster vaccination.1
Yellow fever vaccination is advised for travellers passing through or living in countries in Africa, Central America and South America where yellow fever infection is officially reported. It is also recommended for travel outside of urban areas of countries that do not officially report yellow fever but lie in the yellow fever "endemic zones."1
Laboratory Personnel: Laboratory personnel who might be exposed to virulent yellow fever virus or to concentrated preparations of the 17D vaccine strain by direct or indirect contact or by aerosols also should be vaccinated.1
People ≥60 years old: People 60 years or older should be considered for primary yellow fever vaccination only if travel to areas where yellow fever is considered endemic or transitional cannot be avoided and a high level of protection against mosquito exposure is not feasible. When vaccination of persons over the age of 60 is deemed necessary, an individual risk assessment should be made before vaccination, including an evaluation of the health status of these persons. Additionally, elderly persons who are vaccinated should be carefully monitored for adverse events for 10 days post-vaccination.1,2
About YF-VAX®
YF-VAX® is a live attenuated freeze-dried vaccine prepared by culturing the 17D-204 strain of yellow fever virus in living avian leukosis virus-free (ALV) chicken embryos. YF-VAX® complies with the yellow fever vaccine standards of the World Health Organization (WHO).1
Yellow fever vaccination is required by law upon entry to certain countries irrespective of the traveller’s country of origin and in other countries when travellers are coming from endemic areas. In some cases, vaccination against yellow fever is recommended, although not required by law, e.g., if yellow fever has been reported in the country of destination. In some Asian and other tropical countries where yellow fever does not exist but the transmitting mosquito is found, vaccination is required for arrivals from an endemic country to prevent importation of the disease. Current information on the countries for which an International Certificate of Vaccination or Prophylaxis is required can be obtained from local health departments or from the Public Health Agency of Canada’s (PHAC) Travel Medicine Program website.1
In order to comply with vaccine regulations and to be officially recognized, only designated Yellow Fever Vaccination Centre clinics approved by the PHAC and registered with the World Health Organization (WHO) may carry out yellow fever immunization, which is then recorded on an appropriately validated International Certificate of Vaccination or Prophylaxis.1 According to the Public Health Agency of Canada, the certificate becomes valid 10 days after the vaccine is received and is valid for the life of the person vaccinated.3
Dosage forms and ingredients
YF-VAX® is supplied as a sterile lyophilized powder in single-dose vials with accompanying diluent, available as:
- 5 x 1 dose vials of vaccine and package of 5 x 0.6 mL vials of diluent
The vaccine must be reconstituted immediately before use with the sterile diluent provided.
Each single dose (0.5 mL) is formulated to contain:
Non-medicinal ingredients:
Excipients:
- <7.5 mg sorbitol
- <7.5 mg gelatin
Diluent:
- 0.9% sodium chloride injection USP
Demonstrated immunogenicity response for YF-VAX®1
In studies conducted worldwide since 1962, using 17D vaccines in 2,529 adults and 991 infants and children, the seroconversion rates were between 91% and 100% in all but two studies and never lower than 81%.
In a 2001 double-blind, randomized comparative trial, YF-VAX® was used as a control vs. another 17D-204 vaccine. YF-VAX® was administered to 725 adults ≥18 years old with a mean age of 38 years, and 312 subjects who received YF-VAX® were evaluated serologically. A log neutralization index (LNI) of ≥0.7 was considered evidence of seroconversion.
A month after immunization, 99.3% of subjects had seroconverted with a mean LNI of 2.21.
Results of one clinical trial involving 33 HIV-positive adults indicate that the seroconversion rate to 17D-204 vaccine may be reduced in these patients.
Dosage and administration
YF-VAX® should be administered by the subcutaneous route as a single injection of one dose.
Adults and children 9 months of age or older
Revaccination boosts antibody titre; however, evidence from several studies suggests that yellow fever vaccine immunity persists for at least 30 to 35 years and probably for life.
Injection steps
- Inspect for extraneous particulate matter and/or discolouration before use. If these conditions exist, the product should not be administered.
- Reconstitute the vaccine using only the supplied diluent. Draw the entire volume of the diluent into a syringe of suitable size. Slowly inject the diluent into the vial containing the vaccine, let stand for one or two minutes and then carefully swirl mixture until a uniform suspension is achieved. Avoid vigorous shaking.
- Use vaccine within 60 minutes following reconstitution.
- Swirl the product vial well before withdrawing dose.
- Withdraw the required dose (0.5 mL) of the reconstituted vaccine into a syringe.
- Aseptic technique must be used. Use a separate sterile needle and syringe (or sterile prefilled syringe) for each individual patient. Needles should not be recapped and should be disposed of according to biohazard waste guidelines.
- Before injection, the skin over the site to be injected should be cleansed with a suitable germicide. If a germicide is used to cleanse the skin before immunization, the skin must be allowed to dry thoroughly before the vaccine is administered to prevent inactivation of the vaccine by the germicide.
- Administer the total volume of 0.5 mL subcutaneously.
- Give the patient a permanent personal immunization record and record the immunization history in the permanent medical record of each patient. This should include the name of the vaccine, date given, dose, manufacturer and lot number.
Please see the Product Monograph for complete dosing and administration instructions.
Safety profile
Adverse reactions to 17D yellow fever vaccines were generally mild.
- 2–5% of vaccinees report mild headaches, myalgia, low-grade fevers or other minor symptoms for 5 to 10 days after vaccination
- Fewer than 0.2% of the vaccinees curtail regular activities
Adverse Events Observed with YF-VAX® in a 2001 Double-Blind, Randomized Comparative Trial vs. Another 17D-204 Vaccine in Adults ≥18 Years of Age (N=725)
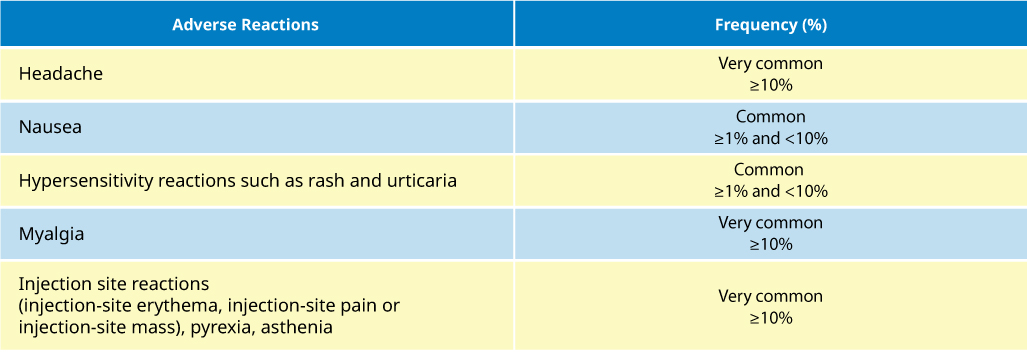
For a complete list of adverse events, please refer to the YF-VAX® Product Monograph.
Resources
Safety information
Contraindications:
- Should not be administered to anyone with a known systemic hypersensitivity reaction to any component of the vaccine, including egg proteins, or its container, or a life-threatening reaction after previous administration of the vaccine or a vaccine containing similar components.
- Persons with congenital or acquired immune deficiency impairing cellular immunity (including those taking immunosuppressive therapies or those with immunosuppression in association with AIDS or other manifestations of HIV, leukemia, lymphoma, thymic disease or generalized malignancy) should not be vaccinated due to risk of encephalitis.
- Alternative means of prevention should be considered in those with thymic dysfunction, as there is evidence that it is an independent risk factor for the development of yellow fever vaccine-associated viscerotropic disease.
- Vaccination of infants <9 months of age is contraindicated because of the increased risk of encephalitis and travel of such persons to rural areas in yellow fever endemic zones or to countries experiencing an epidemic should be postponed or avoided, whenever possible.
Relevant warnings & precautions:
- Syncope has been reported.
- Do not administer by intravascular, intradermal or intramuscular injection.
- Persons with serious acute febrile illness should not be vaccinated until symptoms have abated.
- Isolated cases of yellow fever vaccine-associated viscerotropic disease have been reported to occur within 10 days of vaccination. The risk appears to be higher in those ≥60 years of age.
- Risk of anaphylaxis.
- Immunocompromised persons may not achieve the expected immune response.
- Isolated cases of yellow fever vaccine-associated neurotropic disease have been reported to occur within a month of vaccination.
- Administration of YF-VAX® during pregnancy only if the benefit of preventing the disease is deemed to outweigh the potential risk from the vaccine
- Vaccination of nursing women should be avoided when possible.
For more information:
Visit the Product Monograph for important information relating to adverse reactions, drug interactions, and dosing information which have not been discussed in this piece. The Product Monograph is also available through our medical department. Call us at 1-888-621-1146.
* Comparative clinical significance is unknown.
- Product Monograph: YF-VAX®. Sanofi Pasteur Limited. April 17, 2019.
- National Advisory Committee on Immunization. Yellow fever vaccine: Canadian immunization guide. March 2023. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-25-yellow-fever-vaccine.html.
- Public Health Agency of Canada. Travellers going to yellow fever areas. January 18, 2024. https://www.canada.ca/en/public-health/services/travel-health/yellow-fever/travellers-going-yellow-fever-areas.html
MAT-CA-2201095-v1.0-05/2025







.jpg/jcr:content/image%20(1).jpg)
.jpg/jcr:content/image%20(2).jpg)
.jpg/jcr:content/image%20(3).jpg)