AVAXIM®
For patients 12 years of age and older
A hepatitis A vaccine for children (≥12 years old) and adults
AVAXIM® is indicated for active immunization against infection caused by hepatitis A virus (HAV) in persons 12 years of age and older. AVAXIM® can be used for primary immunization or as a booster following primary immunization with AVAXIM® or other similar hepatitis A vaccines.1
About AVAXIM®
AVAXIM® confers immunity against HAV infection by inducing the production of specific anti-HAV antibodies.*
Dosage forms and ingredients
AVAXIM® is supplied in pre-filled single-dose syringes.
The vaccine is available in packages of:
- 1 x 0.5 mL (single dose) syringe with one needle (1 x 25G x 25 mm).

Each 0.5 mL dose is formulated to contain 160 antigen units (U) of HAV Inactivated (GBM strain).
Non-medicinal ingredients:
2-phenoxyethanol
aluminum hydroxide (expressed as aluminum)
ethanol anhydrous
formaldehyde
Medium 199 Hanks
Manufacturing process residuals: neomycin
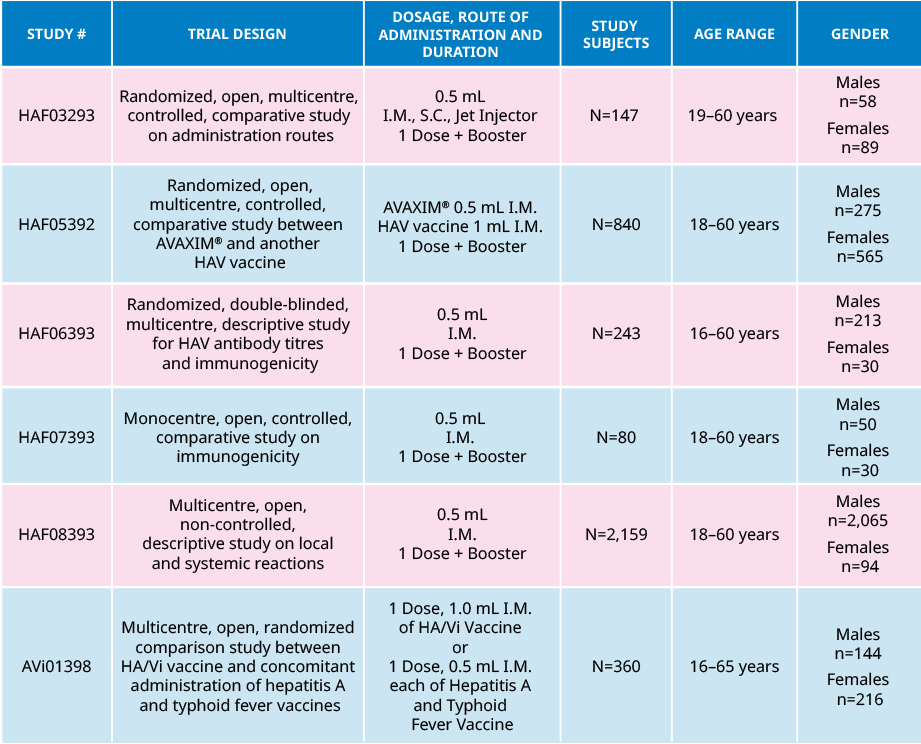
Trial design and study demographic
Summary of Demographics and Study Design of the Trials with AVAXIM®
 |
Immunogenicity data
Immunity appeared shortly after the first injection and persisted for at least 36 months in clinical studies with over 1,000 volunteers
AVAXIM® conferred immunity against HAV by inducing antibody titres greater than those obtained after passive immunization with immunoglobulin.
The studies have shown that:
- Specific humoral antibodies against HAV were elicited after the first injection.
- 14 days after vaccination, >90% of immunocompetent subjects were protected (titres >20 mIU/mL).
- 1 month after the first injection, 100% of the subjects were protected.
- Immunity persisted for at least 36 months and was reinforced after a booster dose.
In comparative trials with another hepatitis A vaccine:
- AVAXIM® demonstrated a superior immunogenicity profile.
- Seroconversion rates at 14 days showed that the immune responses occur more rapidly with AVAXIM®.
(This prompt immune response may be an important consideration when travellers must be vaccinated immediately prior to departure or when post-exposure prophylaxis cannot be done immediately after exposure).
Dosage and administration

Primary immunization
AVAXIM® should be administered as a single injection of 1 dose (0.5 mL) by the intramuscular route.

Second dose
A booster dose should be administered 6 to 36 months after the first dose to confer long-term protection.
Injection steps
-
Inspect for extraneous particulate matter and/or discolouration before use. If these conditions exist, the product should not be administered.
-
Shake the pre-filled syringe well until a uniform, cloudy suspension results.
-
AVAXIM® may be packaged in one of two presentations
-
If a syringe does not have an attached needle, remove the tip cap from the syringe, take the needle from the blister pack and affix to the tip of the pre-filled syringe.
-
If a syringe with attached needle is present, the vaccine is ready to administer.
-
-
Aseptic technique must be used. Use a separate sterile needle and syringe, or sterile disposable unit for each individual patient to prevent disease transmission. Needles should not be recapped and should be disposed of according to biohazard waste guidelines.
-
Administer the vaccine intramuscularly. The preferred site of injection is the deltoid muscle. Do not administer into the buttocks.
-
Give the patient a permanent personal immunization record and record the immunization history in the permanent medical record of each patient. This should contain the name of the vaccine, date given, dose, manufacturer and lot number.
Safety profile
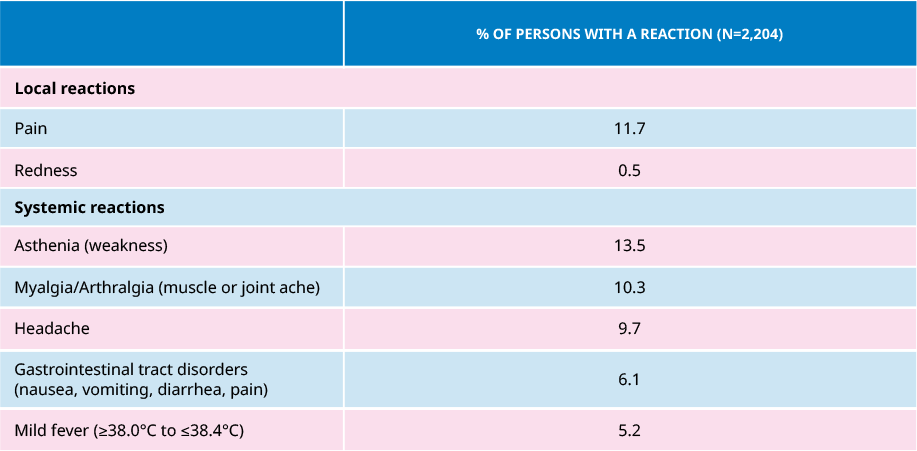
In 6 clinical trials conducted which involved over 2,200 participants, adverse events were usually mild and confined to the first few days after vaccination with spontaneous recovery.
- Mild transient elevation of serum transaminases has been reported on rare occasions.
- The appearance of a nodule at the injection site was observed in very rare cases.
- Adverse reactions were less frequently reported after the booster dose than after the first dose.
- In subjects seropositive to HAV, AVAXIM® was as generally well tolerated as in seronegative subjects.
- The reactions observed in hemophiliac children were identical to those observed in adults.
- In comparative trials with another hepatitis A vaccine, in a total of 423 adults, AVAXIM® demonstrated significantly fewer local reactions after each injection.
Frequency (%) of Reactions Observed After One Dose of AVAXIM®
%20of%20Reactions%20Observed%20After%20One%20Dose%20of%20Avaxim%C2%AE.png) |
Resources
Safety information
CONTRAINDICATIONS:
AVAXIM® is contraindicated in patients who are hypersensitive to this vaccine after previous administration or to any ingredient in the formulation, including any non-medicinal ingredient, or component of the container.
RELEVANT WARNINGS & PRECAUTIONS:
- Do not administer by intravascular injection or intradermally. Ensure the needle does not penetrate a blood vessel. Do not administer into the buttocks.
- As with any vaccine, AVAXIM® may not protect 100% of vaccinated individuals.
- Risk of syncope.
- It is not known if AVAXIM® will prevent hepatitis A if infection is present but not clinically apparent at the time of vaccination.
- Hypersensitivity to formaldehyde and neomycin (and other antibiotics of the same class).
- People with liver disease
- Vaccination should be postponed in cases of an acute or febrile disease.
- AVAXIM® should not be administered to persons with bleeding disorders such as hemophilia or thrombocytopenia, or in persons on anticoagulant therapy, unless the potential benefits outweigh the risk of administration. If the decision is made to administer any product by intramuscular injection to such persons, it should be given with caution, with steps taken to avoid hematoma formation risk following injection.
- In exceptional circumstances (e.g., in patients with thrombocytopenia or in patients at risk of hemorrhage), the vaccine may be administered by the subcutaneous route; however, this may be associated with a higher risk of local reaction including injection site nodule.
- Hypersensitivity reactions may occur following the use of AVAXIM® even in persons with no prior history of hypersensitivity to the product components.
- Immunocompromised persons may not achieve the expected immune response.
- AVAXIM® does not provide protection against infection caused by hepatitis B virus, hepatitis C virus, delta virus, hepatitis E virus, or by other liver pathogens, other than HAV.
- Only for use during pregnancy if clearly needed and following an assessment of risks and benefits.
- Caution in nursing mothers.
- Geriatrics: limited data are available to Health Canada
FOR MORE INFORMATION:
Visit the Product Monograph for important information relating to all warnings and precautions, adverse reactions, drug interactions, and dosing information which have not been discussed in this piece. The Product Monograph is also available through our medical department. Call us at 1-888-621-1146.
I.M.=intramuscular; S.C.=subcutaneous.
* Clinical significance is unknown.
- Product Monograph: AVAXIM®. Sanofi Pasteur Limited. May 14, 2025
MAT-CA-2500526-v1.0-05/2025



.jpg/jcr:content/image%20(1).jpg)
.jpg/jcr:content/image%20(2).jpg)
.jpg/jcr:content/image%20(3).jpg)