Duration of effect: Based on clinical and PK data, a single dose of Beyfortus® offers a minimum duration of protection of at least 5 months.1*
RSV season is here. Beyfortus® is at hand.¹-³
Beyfortus® is an antibody indicated for the prevention of RSV lower respiratory tract disease in infants during their first season.1†
Beyfortus® (nirsevimab injection) is indicated for the prevention of Respiratory Syncytial Virus (RSV) lower respiratory tract disease in:
- Neonates and infants during their first RSV season.
- Children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season, which may include but is not limited to children with: chronic lung disease of prematurity (CLD), hemodynamically significant congenital heart disease (CHD), immunocompromised states, Down syndrome, cystic fibrosis, neuromuscular disease, and congenital airway anomalies.
Study results
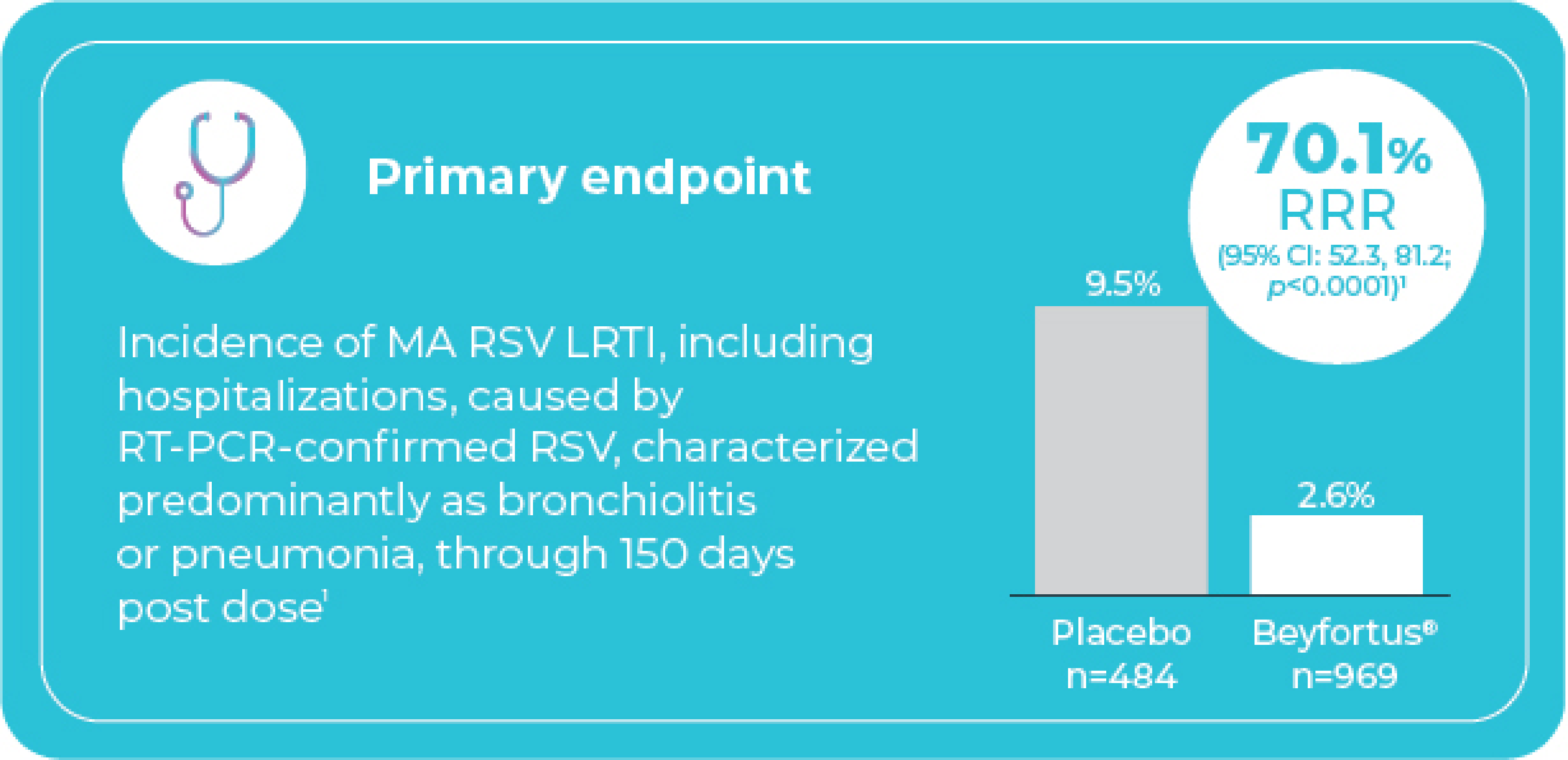
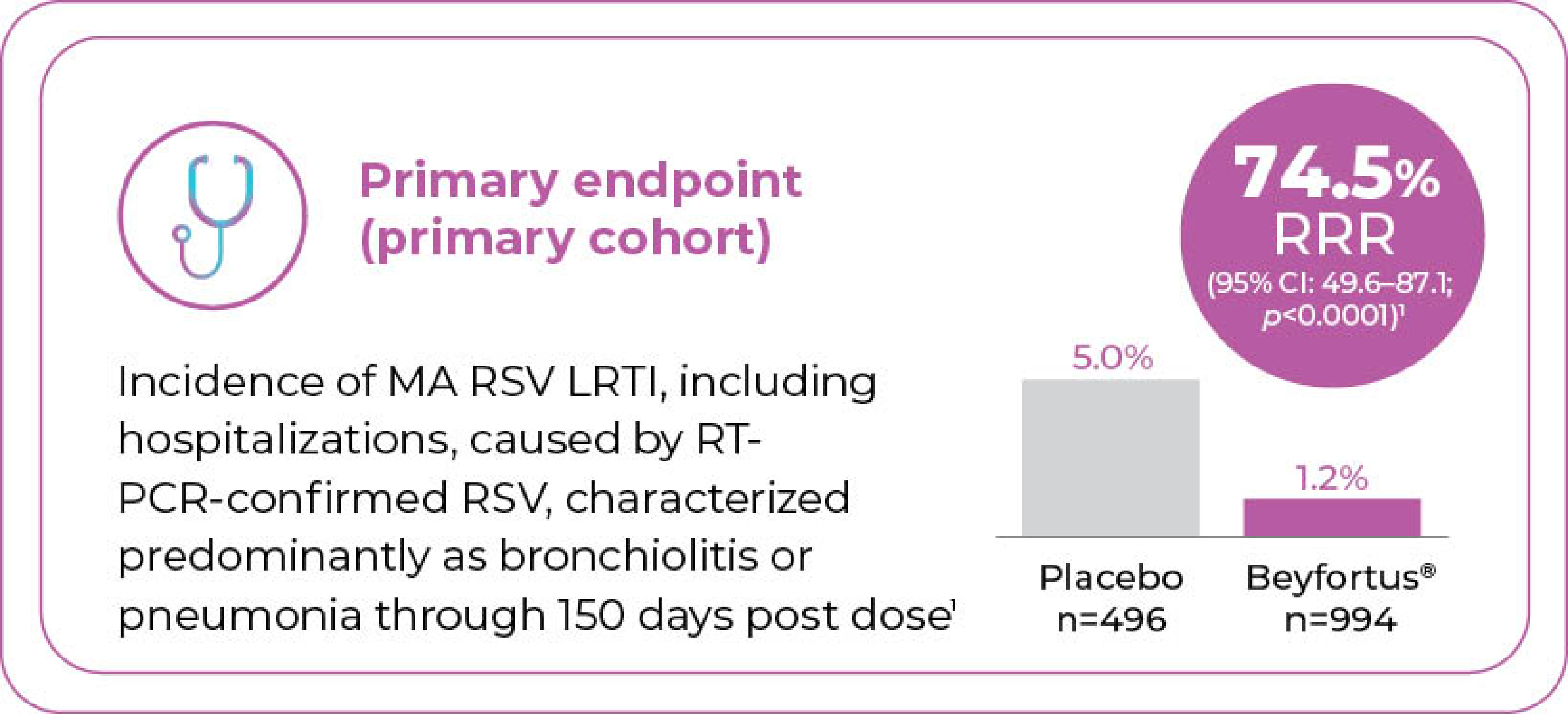
Beyfortus® demonstrated statistically significant efficacy in reducing the relative risk of MA RSV LRTI, including hospitalizations vs. placebo‡§
Study 3: Very and moderately preterm infants¶
entering their first RSV season (GA ≥29 to <35 weeks)
Melody: Term and late preterm infantsˡˡ
entering their first RSV season (GA ≥35 weeks)
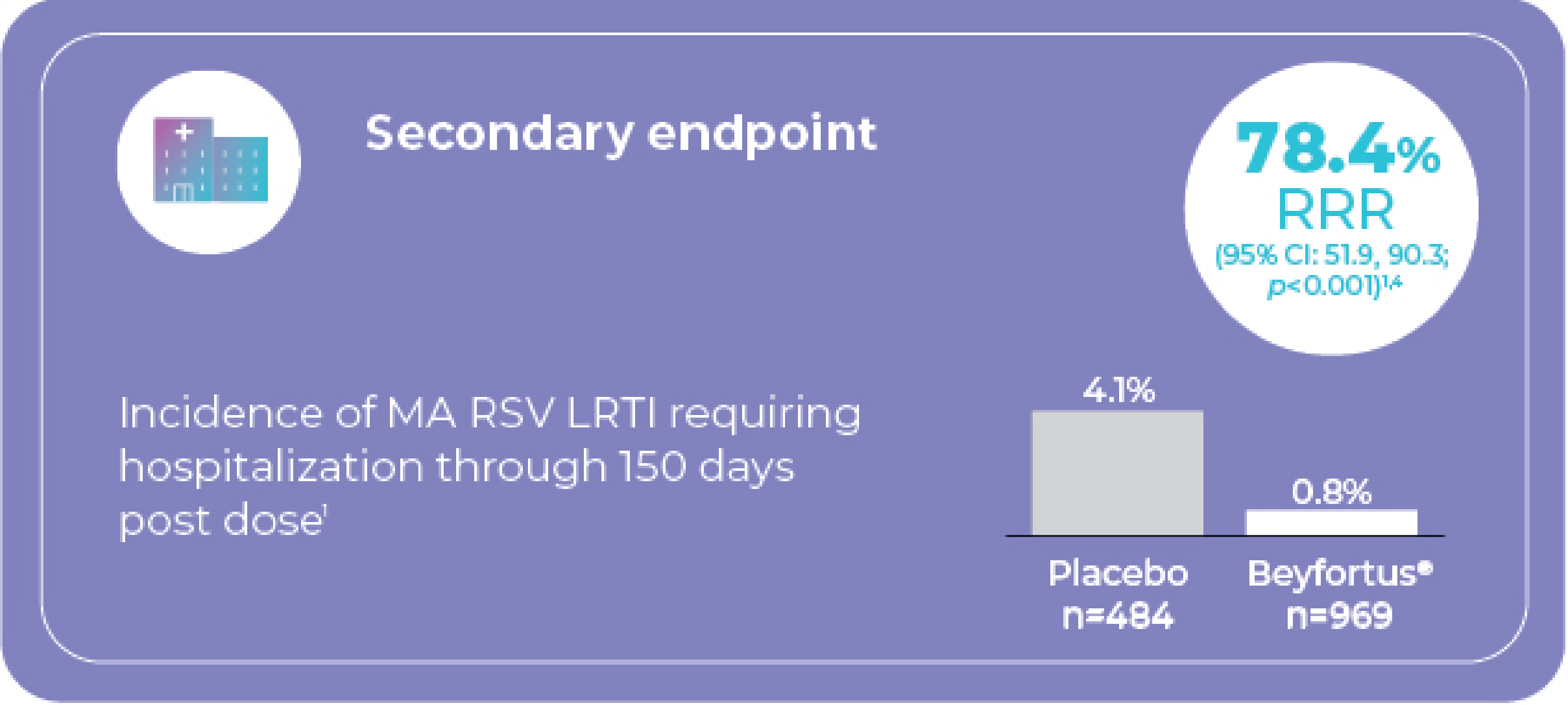
Secondary Endpoint (all subjects)
Incidence of MA RSV LRTI requiring hospitalization through 150 days post injection1
Melody continued to enroll infants following the primary analysis, and overall, 3,012 infants (all subjects) were randomized to receive Beyfortus® (n=2,009) or placebo (n=1,003)1
- 76.8% RRR in incidence of MA RSV LRTI requiring hospitalization through to Day 150 vs. placebo (95% Cl: 49.4, 89.4). Incidence was 0.4% with Beyfortus® vs. 2.0% with placebo1
Beyfortus® was generally well tolerated in clinical trials¹
The most frequent adverse reaction was a rash (0.7% in Beyfortus® and 0.3% in placebo, occurring within 14 days post dose), as well as pyrexia (0.5% vs. 0.6% in placebo) and injection site reactions (0.3% vs. 0% in placebo) within 7 days post dose.
The safety profile for Beyfortus® was generally comparable to placebo in term and preterm infants (GA ≥29 weeks) (data pooled from Study 3 and Melody).
Overall rates of AEs, irrespective of causality, were 84.0% and 82.6% for Beyfortus® and placebo, respectively.
- Majority of AEs were mild or moderate in severity
Most commonly reported AEs (>10% of subjects in either treatment group) for Beyfortus® vs. placebo were:
- Upper respiratory tract infection (31.8% vs. 29.9%)
- Nasopharyngitis (19.0% vs. 21.0%)
- Pyrexia (11.8% vs. 10.3%)
Rates of SAEs, irrespective of causality, were comparable between Beyfortus® and placebo (7.6% and 10.5%).
- No SAEs were determined to be related to Beyfortus®
Most commonly reported SAEs (≥0.5% of subjects in either treatment group) for Beyfortus® vs. placebo were:
- Bronchiolitis (1.3% vs. 2.6%)
- Pneumonia (0.7% vs. 0.9%)
- Gastroenteritis (0.6% vs. 0.4%)
- LRTI (0.6% vs. 0.8%)
- Bronchitis (0.5% vs. 1.0%)
- Urinary tract infection (0.3% vs. 0.5%)
- RSV bronchiolitis (0.2% vs. 0.9%)
- Inguinal hernia (<0.1% vs. 0.5%)
In the study of 918 infants at higher risk of severe RSV disease entering their first RSV season, the safety profile of Beyfortus® (n=614) was similar vs. palivizumab (n=304) and consistent with that seen in studies in healthy term and preterm infants ≥29 weeks GA (Study 3 and Melody)#**
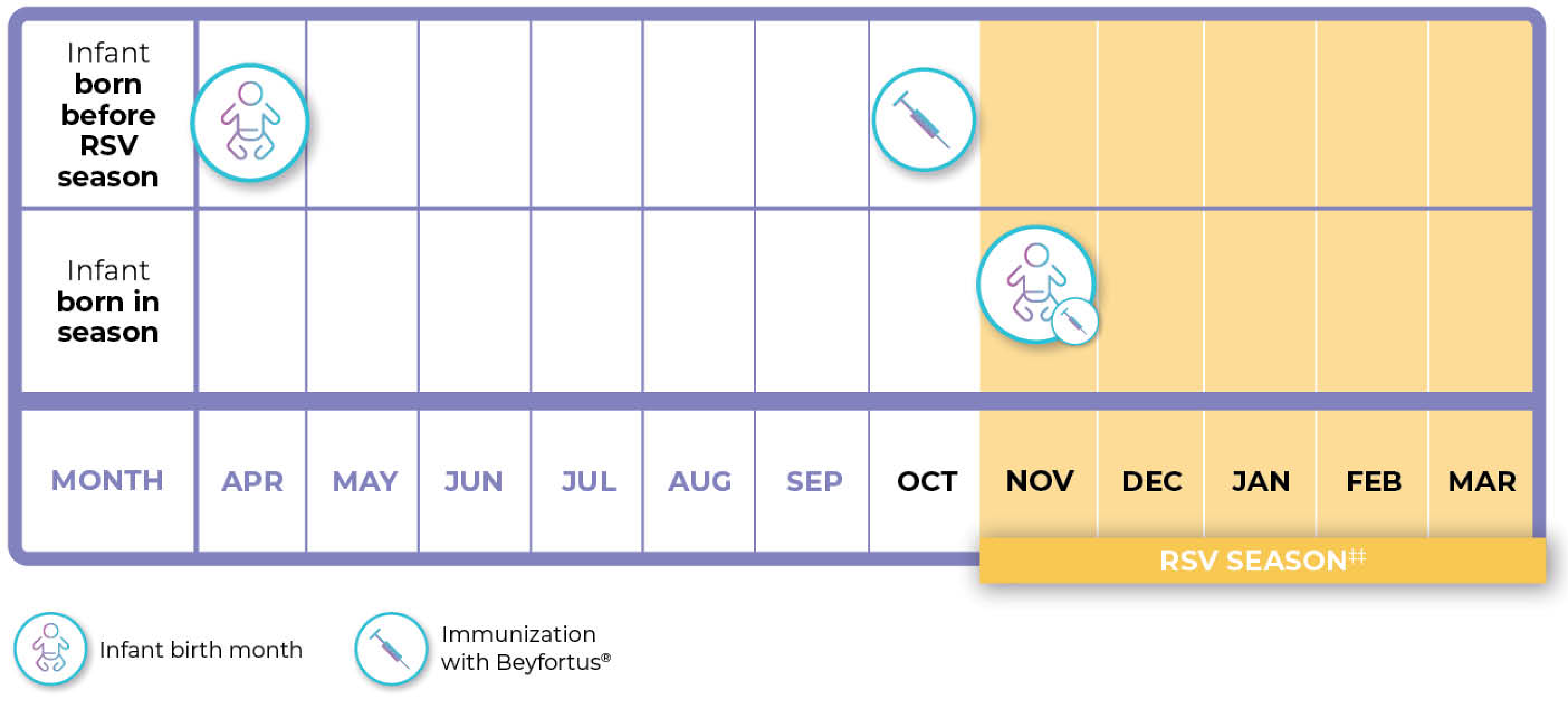
Beyfortus® should be administered prior to commencement of the RSV season, or from birth for infants born during the RSV season¹
Illustrative example only††
Safety information
Clinical use
Safety and efficacy in children older than 24 months of age have not been established. Safety and efficacy in infants with body weight below 1.6 kg have not been established. Dosing in infants with a body weight from 1.0 kg to <1.6 kg is based on extrapolation. The efficacy in infants who remain vulnerable to severe RSV disease during their first or second RSV season has not been directly established and is based on extrapolation of exposure only.
There is limited information available in extremely preterm infants (Gestational Age [GA] <29 weeks) less than 8 weeks of age, and no clinical data are available in infants with a postmenstrual age (gestational age at birth plus chronological age) of 32 weeks. Limited data are available in infants with Down syndrome (n=13), cystic fibrosis (n=5), congenital airway anomalies (n=9), and neuromuscular disease (n=0; not evaluated in clinical trials).
Not indicated in the geriatric population (≥65 years of age).
Relevant warnings and precautions
- Should be given with caution to individuals with thrombocytopenia, any coagulation disorder or to individuals on anticoagulation therapy.
- Serious hypersensitivity reactions have been reported following BEYFORTUS® administration. Anaphylaxis has been observed with human immunoglobulin G1 (IgG1) monoclonal antibodies. If signs and symptoms of anaphylaxis or other clinically significant hypersensitivity reaction occur, immediately discontinue administration and initiate appropriate medicinal products and/or supportive therapy.
- In some individuals with protein-losing conditions, an increased clearance of nirsevimab was observed in clinical trials. Nirsevimab may not provide the same level of protection in individuals with significant protein loss.
- Pregnant and nursing women: not indicated for adults.
For more information
Please refer to the Product Monograph for important information relating to adverse events, drug interactions, and dosing. The Product Monograph is also available by calling 1-800-265-7927.
CI=confidence interval; GA=gestational age; MA=medically attended; LRTI=lower respiratory tract infection; RRR=relative risk reduction; RSV=respiratory syncytial virus; RT-PCR=reverse transcription polymerase chain reaction.
* Clinical significance is unknown.
† Beyfortus® is a human monoclonal antibody.1
‡ Signs of LRTI were defined by having one of the following findings at physical examination indicating lower respiratory tract involvement (e.g., rhonchi, rales, crackles, or wheeze); and at least one sign of clinical severity (increased respiratory rate, hypoxemia, acute hypoxic or ventilatory failure, new onset apnea, nasal flaring, retractions, grunting, or dehydration due to respiratory distress). RSV hospitalization was defined as hospitalization for LRTI with a positive RSV test, or worsening of respiratory status and positive RSV test in an already hospitalized patient.
§ The relative risk reduction and 95% CI were calculated using modified Poisson regression with robust variance including stratification factors (hemisphere and age at randomization).
¶ Study design: Randomized, double-blind, placebo-controlled phase 2b multicentre trial. Study population included 1,453 very and moderately preterm infants (GA ≥29 to <35 weeks) entering their first RSV season. Infants were randomized 2:1 to receive a fixed single intramuscular 50 mg dose of Beyfortus® (n=969) or placebo (n=484). Note: 50 mg is not a recommended dose for infants with body weight ≥5 kg. The recommended dose for infants with body weight ≥5 kg is a single IM dose of 100 mg.
|| Study design: Randomized, double-blind, placebo-controlled phase 3 multicentre trial. Study population included 1,490 term and late preterm infants (GA ≥35 weeks) entering their first RSV season for the primary cohort, with 3,012 term and late preterm infants (GA ≥35 weeks) entering their first RSV season for all subjects. Infants were randomized 2:1 to receive a single intramuscular dose of 50 mg Beyfortus® if <5 kg weight or 100 mg Beyfortus® if ≥5 kg weight at the time of administration (n=994), or placebo (n=496) for the primary cohort, Beyfortus® (n=2,009), or placebo (n=1,003) for all subjects.1
# Infants at higher risk of severe RSV disease included 196 extremely preterm infants (GA <29 weeks) and 306 infants with chronic lung disease of prematurity, and hemodynamically significant congenital heart disease who were term or preterm.1
** There are limited data available in extremely preterm infants (GA <29 weeks) less than 8 weeks of age. No clinical data available in infants with a post-menstrual age (GA at birth plus chronological age) of 32 weeks. Limited data are available in infants with Down syndrome (n=13), cystic fibrosis (n=5), congenital airway anomalies (n=9), and neuromuscular disease (n=0; not evaluated in clinical trials).1
†† Chart represents examples only and not mandatory/recommended specific timings.
‡‡ Shaded area indicates a typical RSV season in a temperate northern hemisphere climate. The RSV season varies by region.5
- Beyfortus® Product Monograph, Sanofi Pasteur Limited, June 14, 2024.
- Bianchini S, et al. Microorganisms 2020;8:2048. doi: 10.3390/microorganisms8122048.
- Pisesky A, et al. PLoS One 2016;11(3):e0150416. doi: 10.1371/journal.pone.0150416.
- Griffin MP, et al. N Engl J Med 2020;383:415–25. doi: 10.1056/NEJMoa1913556.
- Obando-Pacheco P, et al. J Infect Dis 2018;217:1356–64.
© 2025 Sanofi Pasteur Limited. All rights reserved.
MAT-CA-2500072E-v1.0-02/2025




.jpg)
-Bilingual.jpg)
.jpg)

.jpg/jcr:content/image%20(1).jpg)
.jpg/jcr:content/image%20(2).jpg)
.jpg/jcr:content/image%20(3).jpg)