For all age groups
A rabies vaccine for children and adults
IMOVAX® Rabies is indicated for the active immunization of individuals of all age groups to prevent disease caused by the rabies virus. It is indicated for both pre-exposure prophylaxis and post-exposure prophylaxis.1
About IMOVAX® Rabies
Mechanism of action*
Human Diploid Cell Rabies Vaccine (HDCV) together with Rabies Immunoglobulin (RIG) and local treatment are highly effective in preventing rabies in exposed individuals.
- No post-exposure HDCV failures have occurred in Canada or the United States.
- The most important immune response to rabies vaccines is antibodies to the G protein of the viral envelope.
- Pre-exposure vaccination with potent rabies vaccines leads to the development of virus-neutralizing antibodies (VNAs).
- Vaccination also induces production of cytotoxic T cells, which have been shown to protect vaccinated mice in the absence of neutralizing antibodies.
Protection after vaccination is provided by the induction of rabies-neutralizing antibodies. The vaccine also induces memory B-cells that appear to persist for the life span of an individual, as they can be recalled 10 years or more later.
As with any vaccine, immunization with IMOVAX® Rabies may not protect 100% of individuals.
Dosage forms and ingredients
IMOVAX® Rabies is available in:
- single-dose vials of lyophilized vaccine with 1 mL of diluent (sterile water for injection) contained in a disposable syringe with an attached needle
The vial stoppers for the vial and the plunger stoppers and needle shields for the syringes supplied with this product do not contain dry natural latex rubber.
Each 1 mL dose is formulated to contain ≥2.5 international units (IU) of Rabies virus (WISTAR Rabies PM/WI 38 1503-3M Strain).
Non-medicinal ingredients:
human albumin
neomycin
Diluent: sterile water for injection
Demonstrated evidence for IMOVAX® Rabies
Pre-exposure study results
The pre-exposure schedule was evaluated in two randomized, open clinical trials, Study #1 (France) and Study #2 (USA).
In Study #1 (France), 32 persons at occupational risk for rabies received IMOVAX® Rabies on Days 0, 7 and 28 and a booster 1 year later. In Study #2 (USA), adults at occupational risk of rabies were randomized to receive 1 of 4 regimens of rabies vaccine, with a group of 19 receiving IMOVAX® Rabies, 1.0 mL I.M. on Days 0, 7 and 28.1
Rabies Titres Following Pre-exposure Series of IMOVAX® Rabies
Adapted from the Product Monograph.1
CI=confidence interval; GMT=geometric mean titre; I.M.=intramuscular.
In Study #1 (France):
- A 10-year follow-up in 17 patients who received the 3-injection protocol followed by a booster dose at 1 year has shown the maintenance of seroconversion up to 5 years in 96.2%.
- Serology was done annually and individuals who tested negative received a booster dose of vaccine.
Post-exposure study results
Post-exposure to rabies
The post-exposure schedule was evaluated in Study #5 (Iran). In this open trial, 45 persons (3-90 years of age) who had been severely bitten by rabid dogs or wolves received 1.0 mL of IMOVAX® Rabies on each of Days 0, 3, 7, 14, 30 and 90 and heterologous rabies antiserum (40 IU/kg) on Day 0 (44 persons). Post-exposure prophylaxis was begun within hours of or up to 14 days after the bites.1
Rabies Titres Following Post-exposure Series of IMOVAX® Rabies
Adapted from the Product Monograph.1
- All individuals were fully protected against rabies and all developed rabies antibodies.
- All persons, with the exception of a 90-year-old who died from unrelated causes, were healthy 1 year later.
- No rabies developed in the 27 persons with whom contact was maintained for 4 years after rabies exposure.
As with any vaccine, immunization with IMOVAX® Rabies may not protect 100% of individuals.
Simulated post-exposure to rabies
Two clinical trials simulated the post-exposure regimen, Study #3 (RAC09295) and Study #4. In RAC09295, a randomized, modified double-blind, multicentre study, 124 subjects received 5 doses of IMOVAX® Rabies given intramuscularly on Days 0, 3, 7, 14 and 28 and HRIG on Day 0. In Study #4, 64 healthy adults received either HRIG or HRIG and IMOVAX® Rabies to simulate the post-exposure setting.1
Rabies Titres Following Sham Post-exposure Series of IMOVAX® Rabies
Adapted from the Product Monograph.1
CI=confidence interval; HRIG=human rabies immunoglobulin; HTHRIG=heat treated human rabies immunoglobulin.
In Study #3:
- All vaccinees reached a serum antibody titre ≥0.5 IU/mL at the 3rd injection between Day 7 and Day 14.
- Protection was maintained in >98% of subjects 1 year later.
In Study #4:
- In the vaccine groups, the antibody titres rose markedly from Day 7 and reached a maximum value at Day 14.
- All subjects who received RIG and vaccine maintained a protective level through Day 42.
- No significant difference in immunogenicity results between the 2 groups receiving vaccine was observed.
As with any vaccine, immunization with IMOVAX® Rabies may not protect 100% of individuals.
Pediatric immunogenicity data
The pre-exposure schedule (3 doses on Days 0, 7 and 28 by intramuscular route) has been assessed in 112 subjects from 2 to 17 years of age included in the VRV06 study, and in 194 subjects from 5 to 13 years of age included in the RAC03396 study.
After the primary series, all vaccinees reached a serum antibody titre ≥0.5 IU/mL Day 42.
A post-exposure experience in children from Thailand used IMOVAX® Rabies in 50 children aged below 13 years (27 children were below 6 years of age with the youngest 12 months of age). There were no treatment failures.
No efficacy studies were conducted.
Dosage and administration
Adapted from the Product Monograph.1
HDVC=Human Diploid Cell Rabies Vaccine; RFFIT=rapid fluorescent-focus inhibition test; RIG=(human) rabies immunoglobulin;
WHO=World Health Organization.
Reconstitution
Adapted from the Product Monograph.1
Reconstitution Steps
-
Before administration, visually check parenteral drug products for any deviation from normal appearance including container integrity. Inspect the syringe and its package prior to use for evidence of leakage, or a faulty tip seal. If evidence of such defects is observed, do not use the syringe.
- Reconstitute the freeze-dried vaccine in its vial by introducing the diluent into the vial of powder. Gently swirl the contents until completely dissolved. The suspension should be clear or slightly opalescent red to purplish red and free from particles.
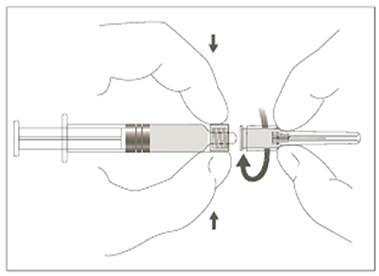
- Without removing the needle from the vial, unscrew the syringe to eliminate negative pressure (as the vial is sealed under vacuum). Reattach the needle remaining in the vial to the syringe (see Attaching the needle to the Luer-LokTM syringe visual below).
- Withdraw the total contents of the vial into the syringe.
- Unscrew the reconstitution needle and replace it with a sterile needle (see Attaching the needle to the Luer-LokTM syringe visual below) of a proper length for intramuscular injection of your patient.
- The reconstituted vaccine should be used immediately (see Administration section below).
- After use, any remaining vaccine and container must be disposed of safely, according to biohazardous waste guidelines.
Attaching the needle to the Luer-LokTM syringe
Administration
Administer the vaccine intramuscularly.
- For adults and children, the vaccine should always be administered in the deltoid area.
- In infants and small children, the anterolateral aspect of the thigh is also acceptable.
- The gluteal area should never be used for injections because administration of rabies vaccine in this area results in lower neutralizing antibody titres.
For information on vaccine administration, see the current edition of the Canadian Immunization Guide.
Under no circumstances should vaccine be administered in the same syringe or at the same site as RIG.
Safety profile
IMOVAX® Rabies (Rabies Vaccine Inactivated [DCO]) has been studied in randomized controlled trials in both children (N=199) using pre-exposure schedule (3 doses, I.M. plus booster at 1 year) and adults (N=124) using post-exposure schedule (5 doses, I.M.).
The most frequent (≥10%) adverse events were injection site pain, headache, malaise and myalgia.
Adverse Reactions Reported in Clinical Trial of Patients Following Any Dose of IMOVAX® Rabiesa,b
Adapted from the Product Monograph.1
HRIG=human rabies immunoglobulin.
a A pooled analysis has been performed on 4 randomized, controlled, observer-blind clinical studies sharing the same safety standards, integrating data from 401 subjects (113 children and adolescents from 2 through 17 years of age and 288 adults from 18 through 65 years of age). In 2 studies in adults, the subjects received HRIG concurrently with the first dose of IMOVAX® Rabies. The adverse reactions were generally of mild intensity and appeared within 3 days after vaccination. Most reactions resolved spontaneously within 1 to 3 days after onset.
b This table presents the frequencies of solicited adverse reactions (recorded within 7 days) and unsolicited related adverse events (recorded within 28 days), reported following any dose of IMOVAX® Rabies.
For a complete list of adverse events, please refer to the IMOVAX® Rabies Product Monograph.
Resources
Safety information
Clinical use:
Geriatrics (≥65 years of age): There are no adequate and well-controlled studies of IMOVAX® Rabies in the geriatric population.
Contraindications:
- Pre-exposure prophylaxis should not be administered to persons who are hypersensitive to this vaccine or to any ingredient in the formulation or component of the container. Persons who are at high-risk of contracting rabies disease and who have a hypersensitivity to the vaccine or one of its components may be referred for an evaluation by an allergist.
- There are no definite contraindications to the use of IMOVAX® Rabies in the post-exposure situation; however, care should be taken if the vaccine is to be administered to persons who are hypersensitive to rabies vaccine or to any ingredient in the formulation or component of the container. Local public health should be consulted if questions arise about the need for post-exposure treatment and expert opinion should be sought in the management of these individuals.
Most serious warnings & precautions:
- In adults and children the vaccine should be injected into the deltoid muscle. In infants and small children the mid-lateral aspect of the thigh may be preferable. There have been reports of possible vaccine failure when the vaccine has been administered in the gluteal area.
- This vaccine must not be used subcutaneously or intradermally. Special care should be taken to ensure that the product is not injected into a blood vessel.
Other relevant warnings & precautions:
- It is very important to complete the series of rabies vaccinations on time. Cases of rabies have been reported when the approved schedule was not followed.
- As with any vaccine, IMOVAX® Rabies may not protect 100% of vaccinated individuals.
- Pre-exposure immunization with IMOVAX® Rabies should be deferred in the presence of any acute illness, including febrile illness.
- Local and/or mild systemic reactions may occur after vaccine injection but these are usually transient and do not contraindicate continuing immunization.
- Interchanging IMOVAX® Rabies with other rabies vaccines during a pre- or post-exposure series is not recommended because of a lack of data on the safety profile and efficacy of such a regimen.
- In both pre-exposure and post-exposure immunization, the full 1.0 mL dose should be given intramuscularly. Failures have occurred abroad when some deviation was made from the recommended post-exposure treatment protocol or when less than the currently recommended amount of antirabies sera was administered.
- This product carries an extremely remote risk for transmission of viral diseases.
- Caution must be exercised when the vaccine is administered to subjects with hypersensitivity to neomycin and other antibiotics of the same class.
- Intramuscular injections should be given with care in persons with coagulation disorders or on anticoagulant therapy.
- Corticosteroids, immunosuppressive agents, and immunosuppressive illnesses can interfere with the development of active immunity after vaccination.
- In immunocompromised individuals with congenital or acquired immunodeficiency, the immune response to the vaccine may be inadequate. Therefore, it is recommended to monitor serologically RVNA (Rabies Virus Neutralizing Antibodies) level in such individuals to ensure that an acceptable immune response has been induced. Additional doses may be given as necessary.
- In the case of pre-exposure immunization, a significant increase has been noted in “immune complex-like” reactions in persons receiving booster doses of IMOVAX® Rabies.
- Post-immunization antibody titre determination may be advisable for those anticipating frequent exposure or whose immune response may be reduced by illness, medication or advanced age.
- Syncope can occur following or before any vaccination
- The potential risk of apnea and the need for respiratory monitoring for 48–72 hours should be considered when administering the primary immunization series to very premature infants (born ≤28 weeks of gestation) and particularly for those with a previous history of respiratory immaturity.
- Local reactions at injection site such as pain, erythema, swelling, induration and bruising may occur.
- The safety of rabies vaccines in pregnancy has not been established. IMOVAX® Rabies should be given to a pregnant woman only if clearly needed.
- It is not known whether this vaccine is excreted in human milk. Caution must be exercised when pre-exposure vaccine is administered to a nursing mother. Due to the severity of the disease, lactation is not a contraindication in the post-exposure case.
For more information:
Visit the Product Monograph for important information relating to adverse reactions, drug interactions, and dosing information which have not been discussed in this piece. The Product Monograph is also available through our medical department. Call us at 1-888-621-1146.
* Clinical significance unknown.
- Product Monograph: IMOVAX® Rabies. Sanofi Pasteur Limited. July 2, 2024.
MAT-CA-2201022-v1.0-05/2025




.jpg/jcr:content/image%20(1).jpg)
.jpg/jcr:content/image%20(2).jpg)
.jpg/jcr:content/image%20(3).jpg)