Introducing MenQuadfi®
Used for protection against MenACWY serogroups for individuals 12 months and older, including geriatric use1
MenQuadfi® is indicated for active immunization for the prevention of invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, W and Y in individuals 12 months of age and older. MenQuadfi® does not prevent N. meningitidis serogroup B disease.1
Before you prescribe this vaccine, please refer to the Product Monograph.
Consider MenQuadfi®:
- The only MenACWY tetanus toxoid conjugate vaccine indicated in geriatric use.1,2
- As a booster: A single dose of MenQuadfi® can be used as a booster in adolescents and adults at continued risk of meningococcal disease (if at least 4 years have elapsed since a prior dose of MenACWY conjugate vaccine). There are no data available yet to indicate the need for or timing of a booster dose of MenQuadfi® for individuals who have been primed with MenQuadfi®.1
- Available in a ready-to-use formulation with no reconstitution required.1
About MenQuadfi®
MenQuadfi® is a clear, colourless sterile liquid vaccine administered by intramuscular injection that contains Neisseria meningitidis serogroup A, C, W and Y capsular polysaccharide antigens individually conjugated to tetanus toxoid protein prepared from cultures of Clostridium tetani.
To prepare the polysaccharides for conjugation, serogroup A is activated with carbonyldiimidazole (CDI), derivatized with adipic acid dihydrazide (ADH), and purified by diafiltration. Serogroups C, W, and Y are depolymerized, activated with periodate, and purified by diafiltration.
Dosage forms and ingredients
MenQuadfi® is supplied in single-dose vials.
The vaccine is available as the following:
- 1 x 0.5 mL dose vial
- 10 x 0.5 mL dose
Each 0.5 mL dose is formulated to contain:
- 10 mcg each of meningococcal A, C, Y and W polysaccharide concentrate conjugated to a total of approximately 55 mcg of a tetanus toxoid protein carrier
Non-medicinal ingredients:
- 3.35 mg sodium chloride
- 0.3 mL 50 mM sodium acetate, pH 6.0
- QS to 0.5 mL water for injection
MenQuadfi® is preservative-free and no adjuvant is added during manufacture. The chlorobutyl stopper of the vial does not contain natural latex.
- MenQuadfi® is available for use in individuals aged 12 months and older for protection against IMD caused by serogroups A, C, W and Y.
- Available in a ready-to-use formulation.1
- MenQuadfi® uses a tetanus toxoid carrier protein.1
MenQuadfi® was assessed in 7 pivotal trials*, including head-to-head clinical trials in individuals 12 months and older1
Non-inferiority of MenQuadfi® versus comparators was demonstrated in all studies for all 4 serogroups1*

Toddlers
(12–23 months)
MenQuadfi® vs Nimenrix†
MET 51: Phase III study in vaccine-naïve and vaccine-primed population
MenACWY-TT is a meningococcal group A, C, W-135 and Y conjugate vaccine

Children
(2–9 years, with subgroups 2–5 & 6–9)
MenQuadfi® vs Menveo‡
MET35: Phase III study in vaccine-naïve population MenACWY-CRM is a meningococcal group MenACWY-CRM is a meningococcal group A, C, W-135 and Y conjugate vaccine

Adolescents
(10–17 years, 12 years of age received MenQuadfi® alone)
MenQuadfi® vs Menveo‡
MET50: Phase II study in children and adolescents MenACWY-CRM is a meningococcal group A, C, W-135 and Y conjugate vaccine

Adults
(10–55 years, with subgroups 10–17 &
18–55)
MenQuadfi® vs Menactra§
MET43: MenACWY is a polysaccharide diphtheria toxoid conjugate vaccine (groups A, C, Y and W-135)

Older adults
(≥56 years)
MenQuadfi® vs Menomune¶
MET49: Phase III study
MenA/C/Y/W-135 is a meningococcal polysaccharide vaccine (groups A, C, Y, W-135 combined)
Non-inferiority as assessed by vaccine seroresponse rates was demonstrated if the lower limit of the 2-sided 95% CI of the difference was >-10% for all 4 serogroups.1
For complete study designs and efficacy data, please see the Product Monograph.
*7 pivotal trials included trials with MenQuadfi® alone, with concomitant vaccines (+/- MenQuadfi®), or with a comparator meningococcal vaccine.
† Nimenrix – MenACWY-TT [Meningococcal Polysaccharide Groups A, C, W-135 and Y Conjugate Vaccine]
‡ Menveo – MenACWY-CRM [Meningococcal (Groups A,C, W-135, and Y) Oligosaccharide Diphtheria CRM197 Conjugate Vaccine]
§ Menactra – MenACWY-DT [Meningococcal (Groups A,C, W-135, and Y) Polysaccharide Diphtheria Toxoid Vaccine
|| Menomune – MenA/C/Y/W-135 [Meningococcal Polysaccharide Vaccine, Groups A, C, Y and W-135 Combined]
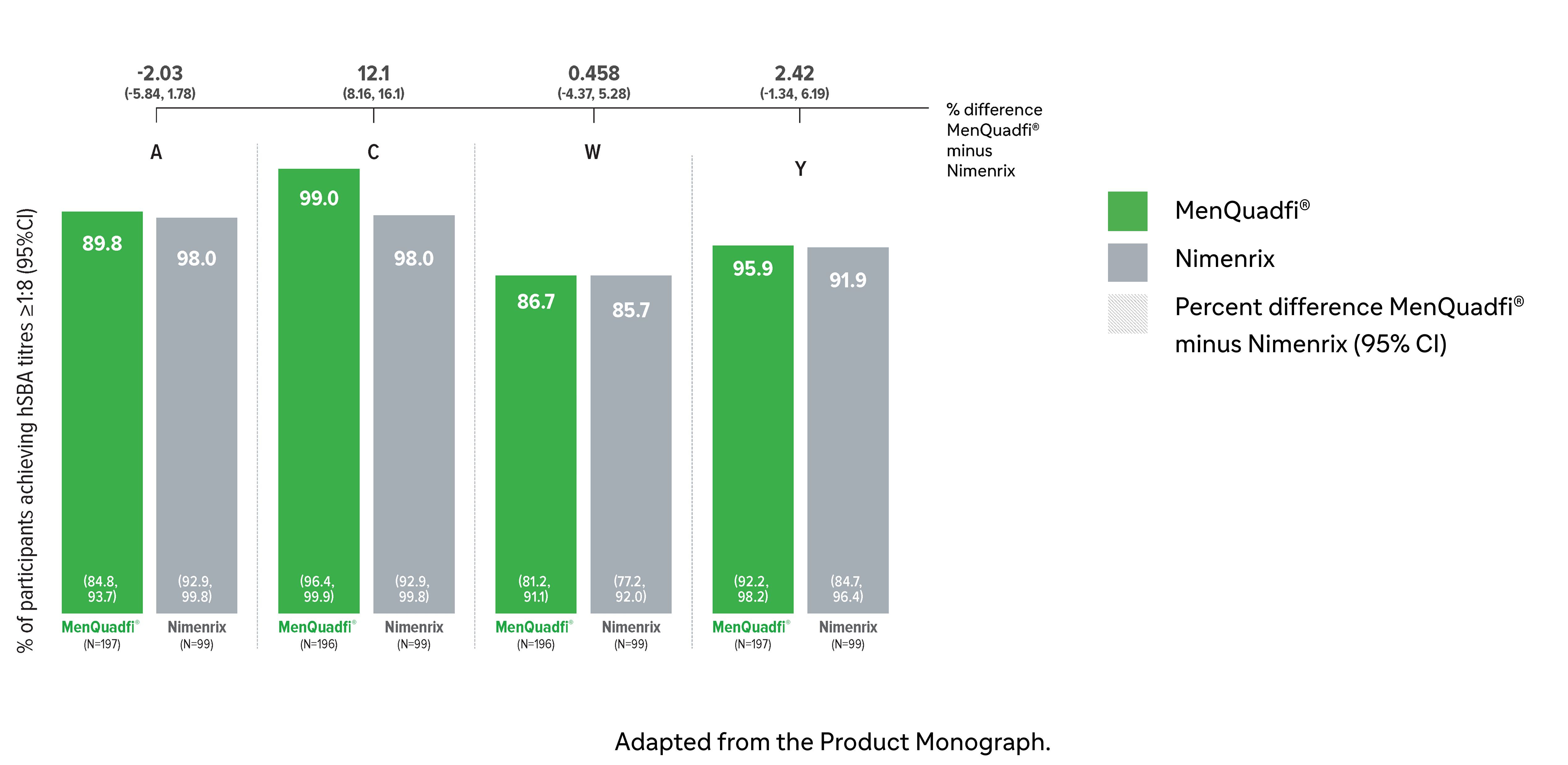
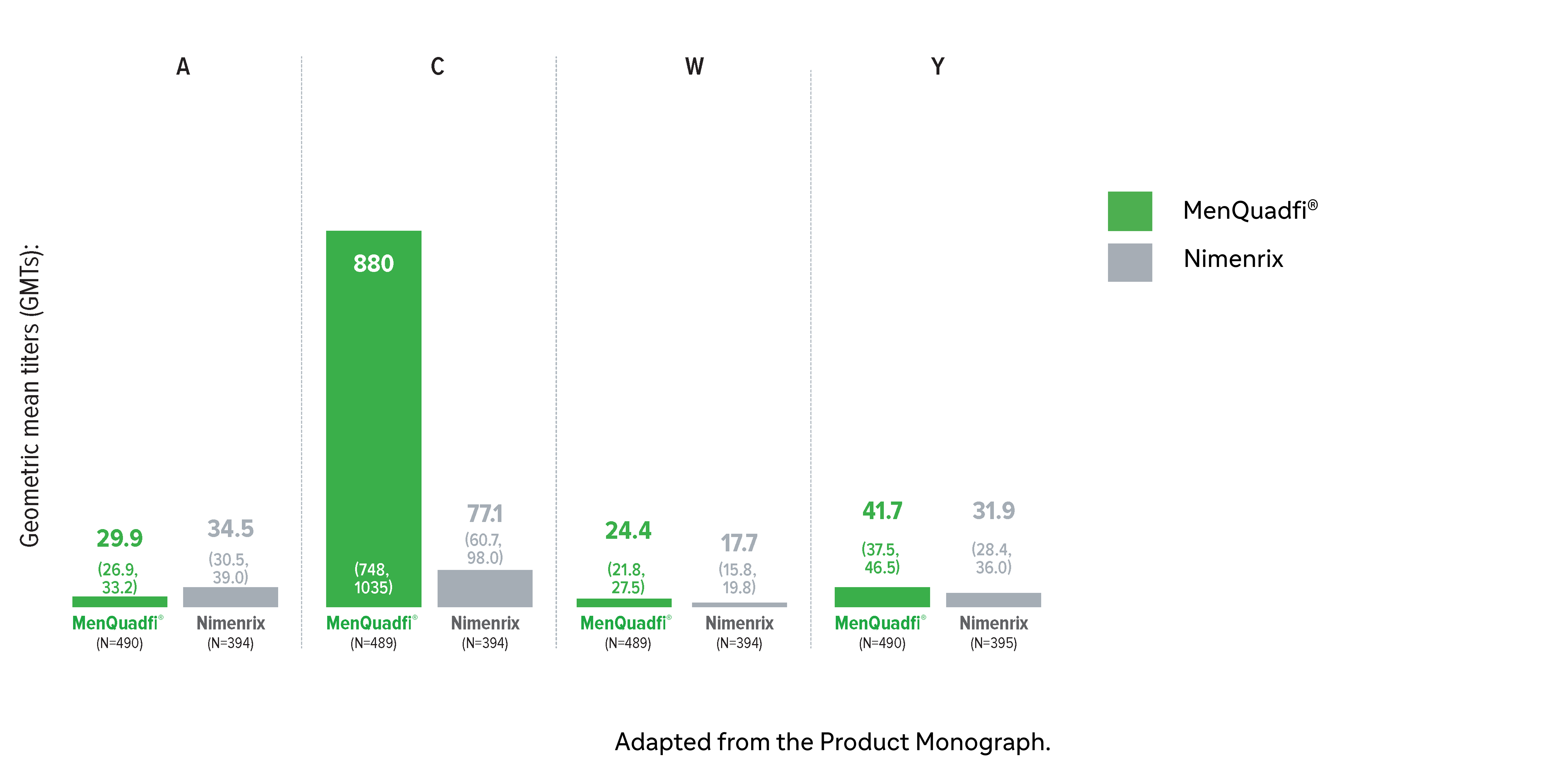
Immunogenicity in toddlers (12-23 months)
Non-inferiority of immune response was demonstrated for MenQuadfi® vs Nimenrix for all serogroups (MET51 study)1*
Non-inferiority was based on percentage of subjects achieving seroprotection (a post-vaccination hSBA titer ≥1:8 at Day 30)
MenQuadfi® vs Nimenrix†
Percentage of participants achieving seroprotection (95% CI)
MenQuadfi® vs Nimenrix†
CI = confidence interval; hSBA = serum bactericidal assay using human complement. N = number of subjects in the per protocol analysis set with valid serology results.
* This study was conducted in participants who were either meningococcal vaccine naive or had been primed with monovalent MenC vaccines (MenC-TT or MenC-CRM) in the first year of life. The participants were randomized to receive either a single dose of MenQuadfi® or the licensed Nimenrix vaccine.1
† Seroprotection rate (primary end point) for each serogroup: the proportion of participants with an hSBA postvaccination titer ≥1:8. Overall non-inferiority would be demonstrated if the lower limit of the 2-sided 95% CI is > -10% for all four serogroups.1
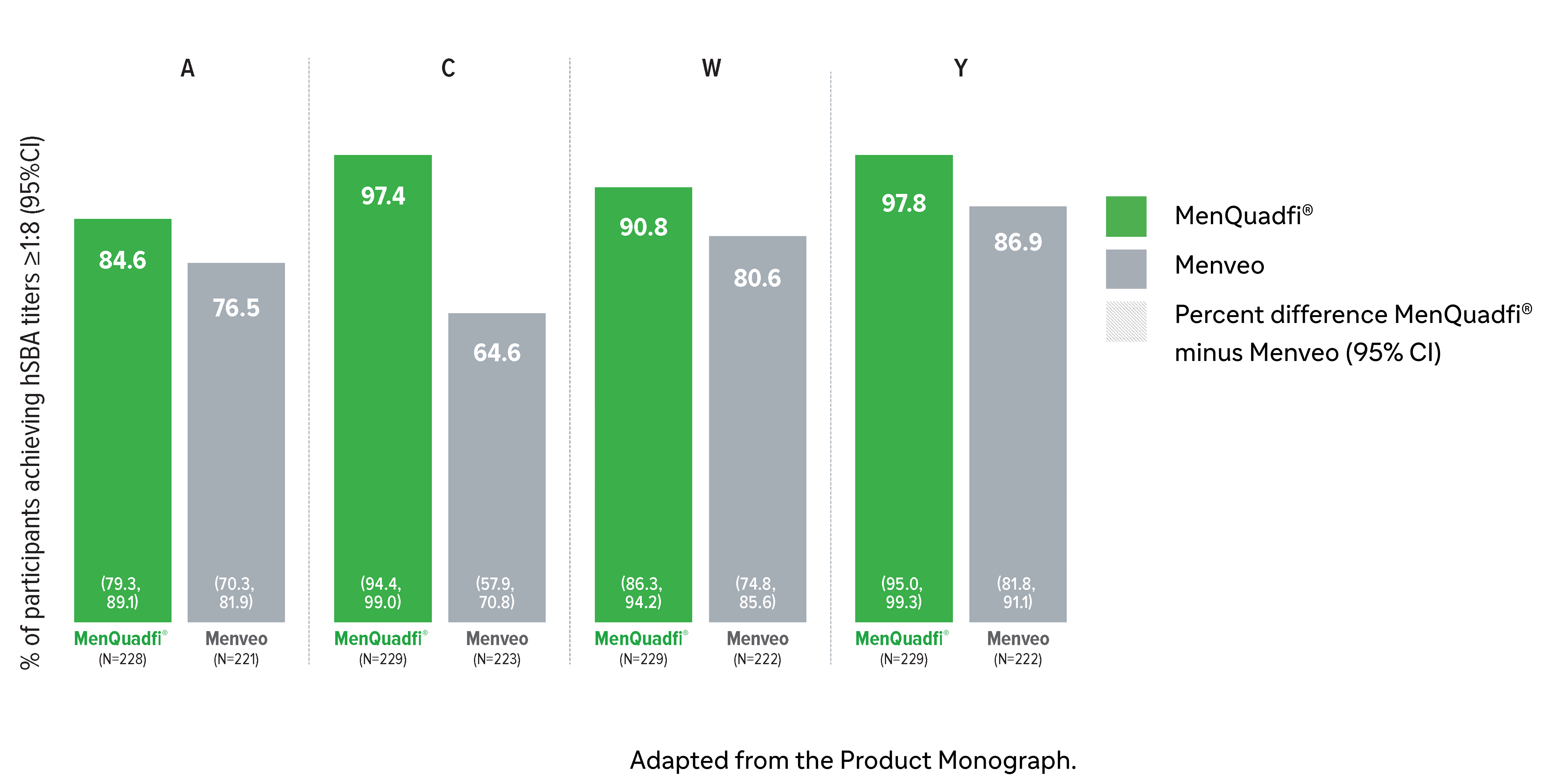
Immunogenicity in children (2-9 years)
Non-inferiority of immune response was demonstrated for MenQuadfi® vs Menveo for all serogroups (MET35 study)1
MenQuadfi® vs Menveo1
Percentage of participants achieving seroprotection (95% CI)
Percentage of participants achieving seroprotection (95% CI)
MenQuadfi® vs Menveo†

CI = confidence interval; hSBA = serum bactericidal assay using human complement. N = number of subjects in the per protocol analysis set with valid serology results.
* Randomized, multicenter, active-controlled, modified, double-blind clinical study comparing MenQuadfi® to Menveo in participants 2–9 years of age.1
† Seroprotection rate (primary end point) for each serogroup: the proportion of participants with an hSBA postvaccination titer ≥1:8. Overall non-inferiority would be demonstrated if the lower limit of the 2-sided 95% CI is > -10% for all four serogroups.1
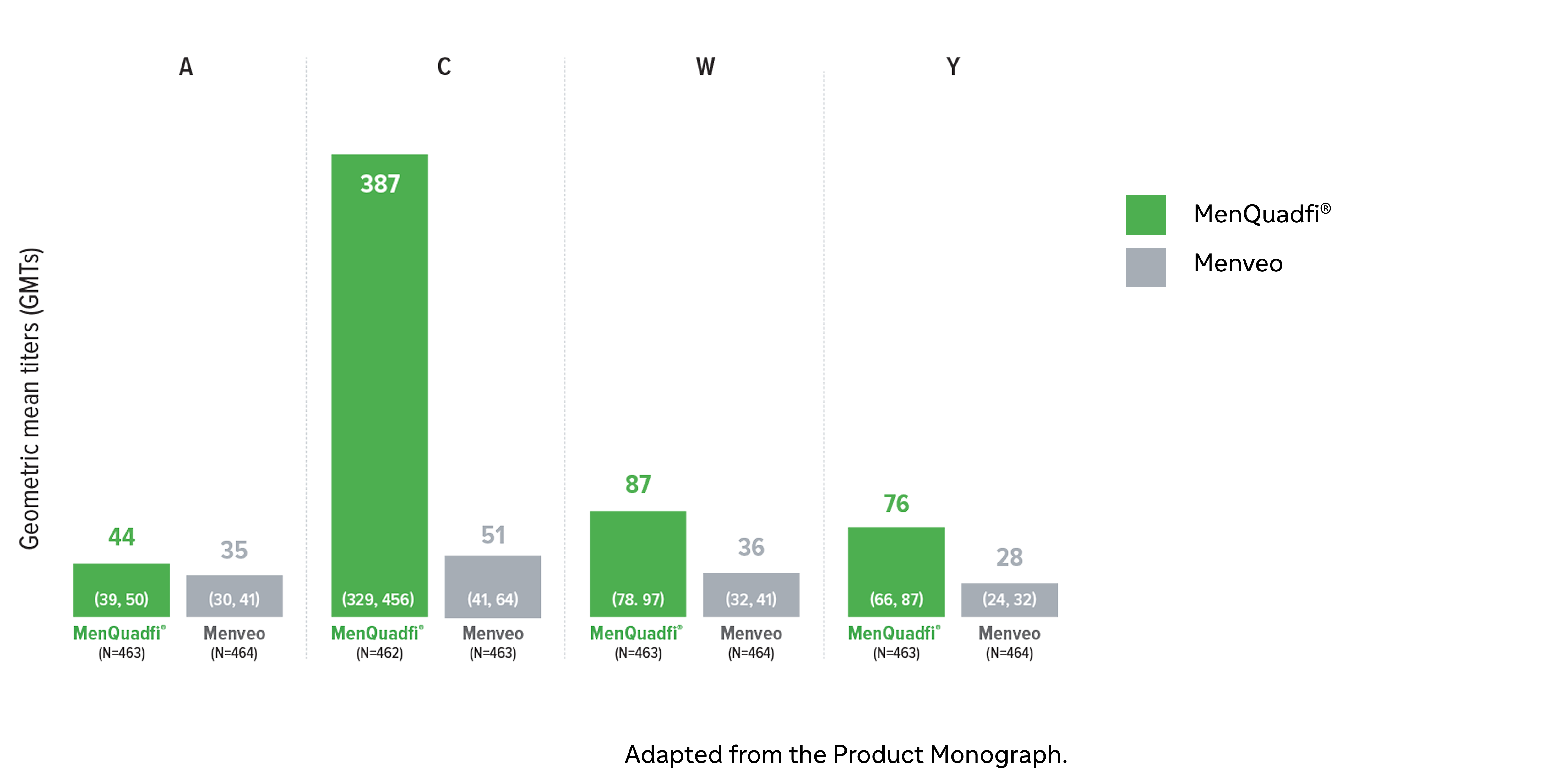
Immunogenicity in adolescents (10-17 years)
Non-inferiority of immune response was demonstrated for MenQuadfi® vs Menveo for all serogroups (MET50 study)1
MenQuadfi® vs Menveo1
Percentage of participants achieving seroprotection (95% CI)
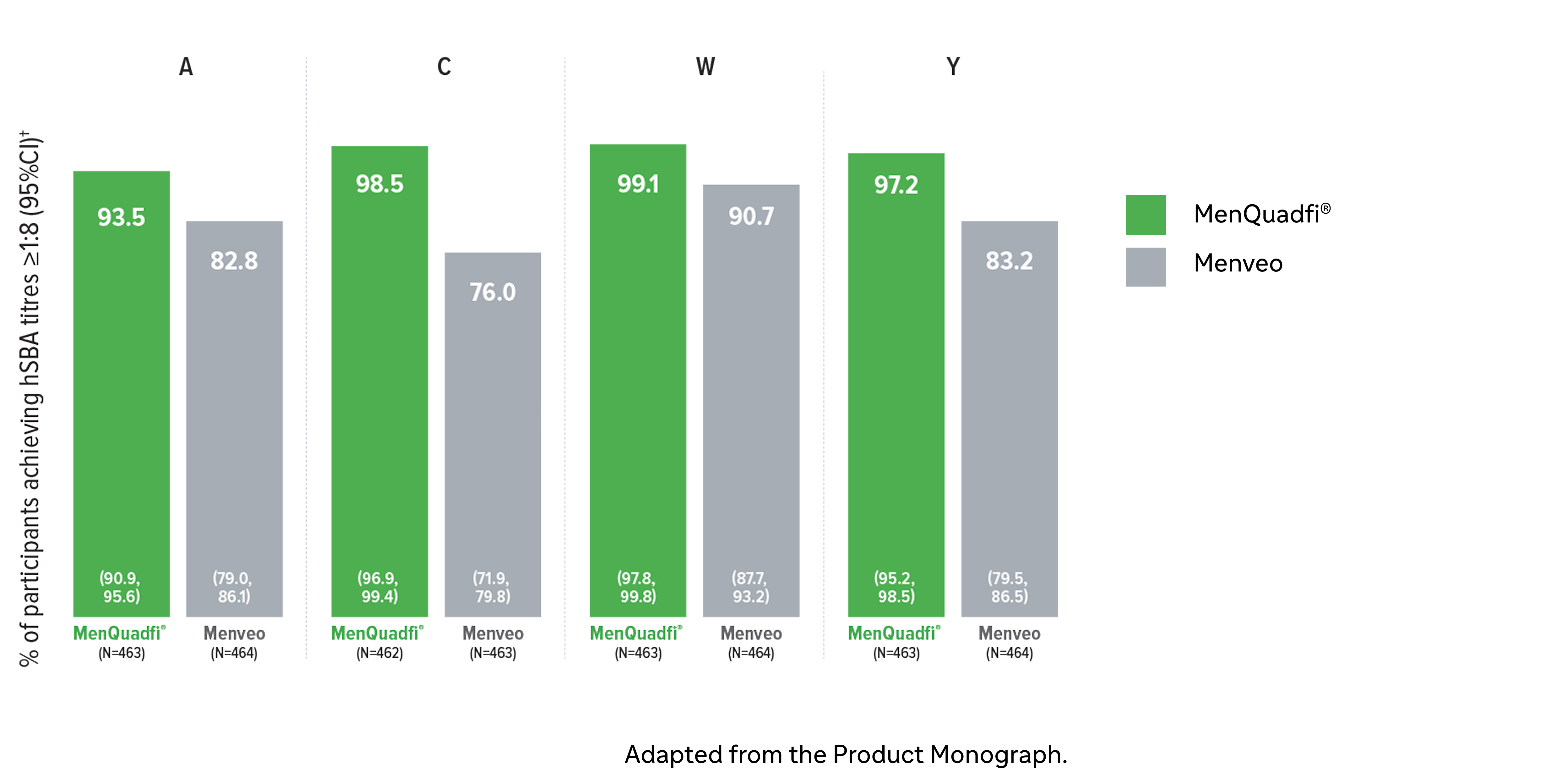
Percentage of participants achieving seroprotection (95% CI)
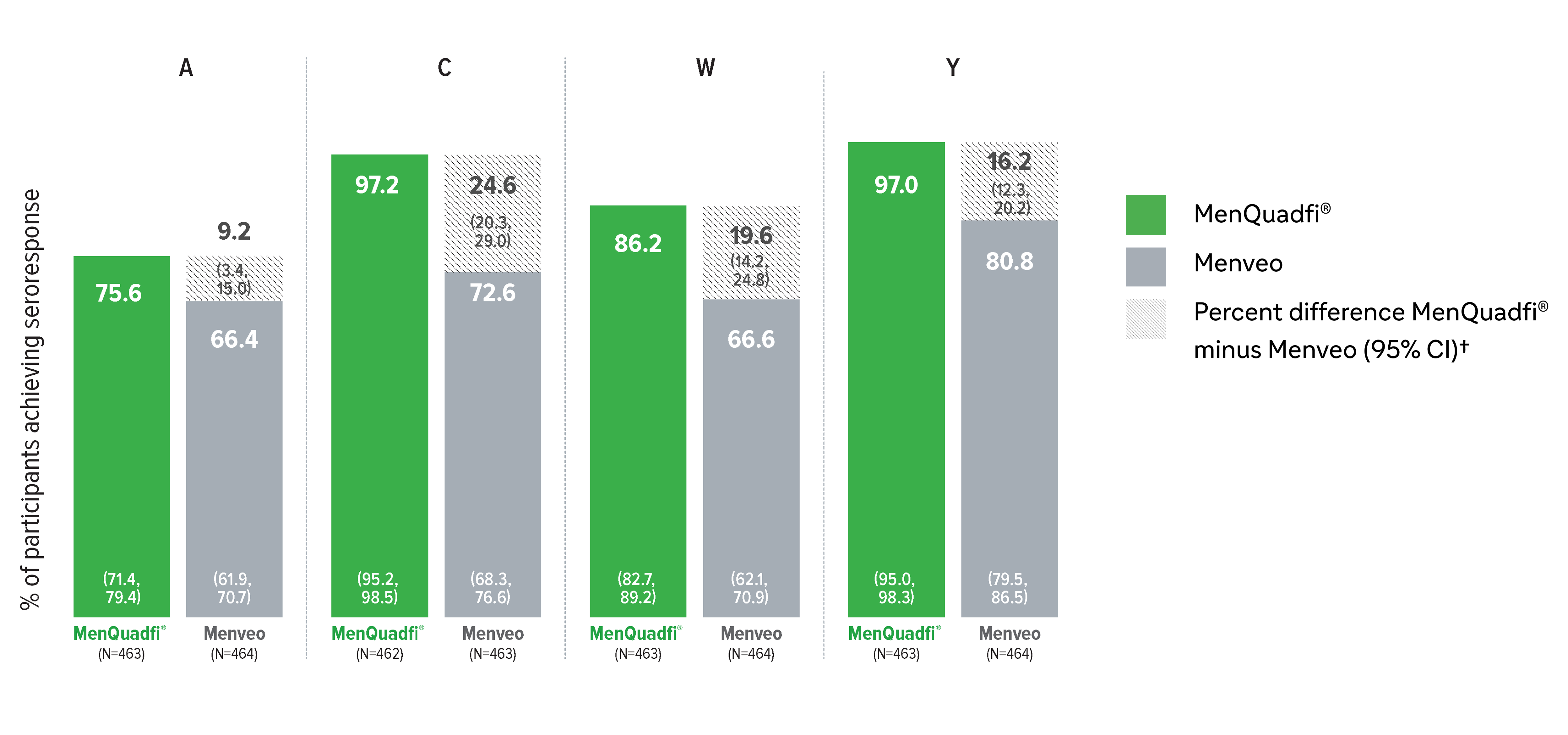
Geometric mean titers (GMTs)
* Randomized, multicenter, active-controlled, clinical study comparing MenQuadfi® to Menveo in participants 10-17 years of age. Conducted in healthy meningococcal vaccine naive male and female participants following administration with either MenQuadfi® alone; Menveo alone; MenQuadfi® co-administered with Adacel (Tdap) and Gardasil (a quadrivalent HPV); or Adacel (Tdap) and Gardasil (a quadrivalent HPV) alone.1
† Seroprotection rate (primary end point) for each serogroup: the proportion of participants with an hSBA postvaccination titer ≥1:8. Overall non-inferiority would be demonstrated if the lower limit of the 2-sided 95% CI is > -10% for all four serogroups.1
CI = confidence interval; hSBA = serum bactericidal assay using human complement. N = number of subjects in the per protocol analysis set with valid serology results.
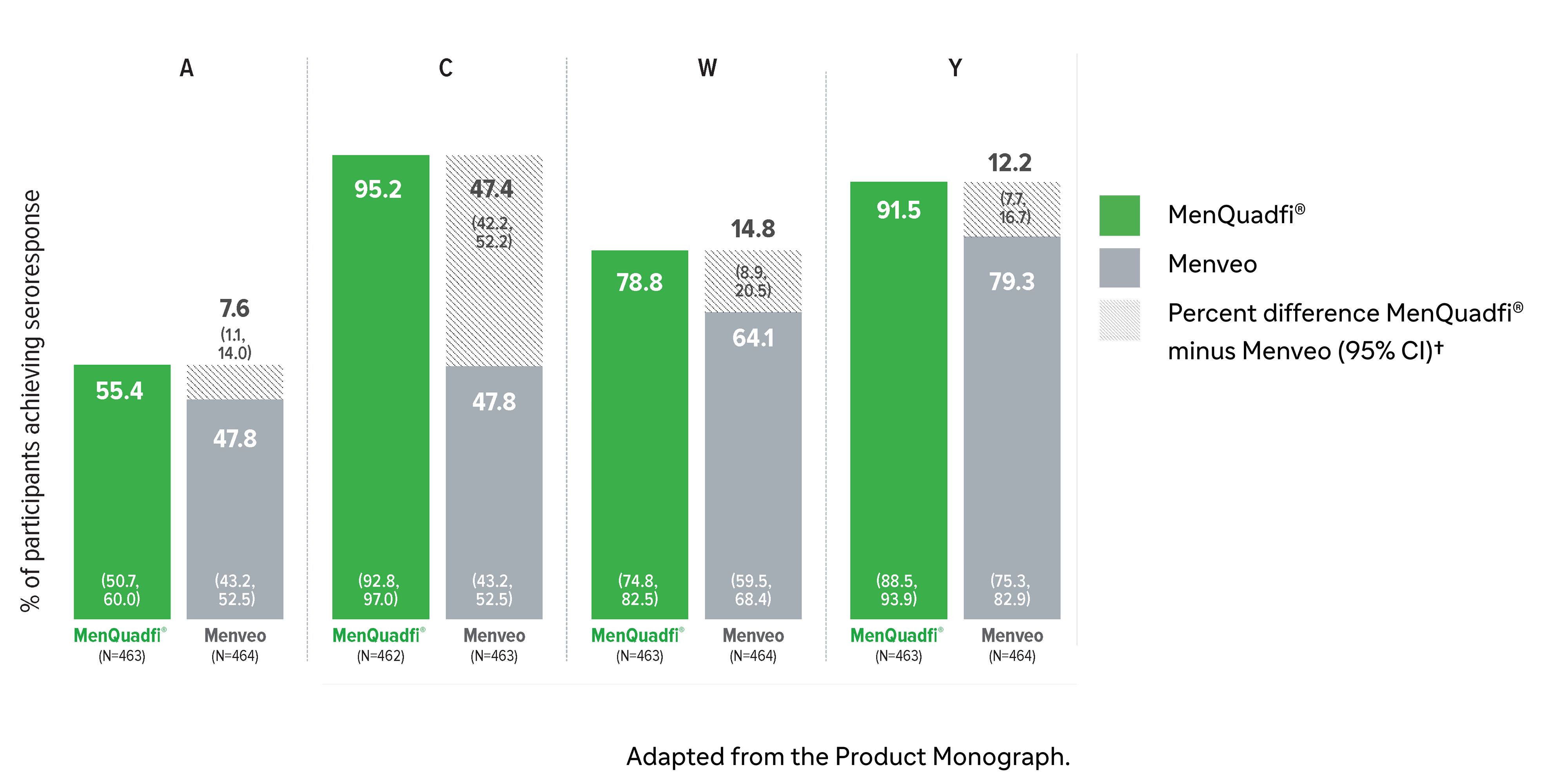
Immunogenicity in adults (18-55 years)
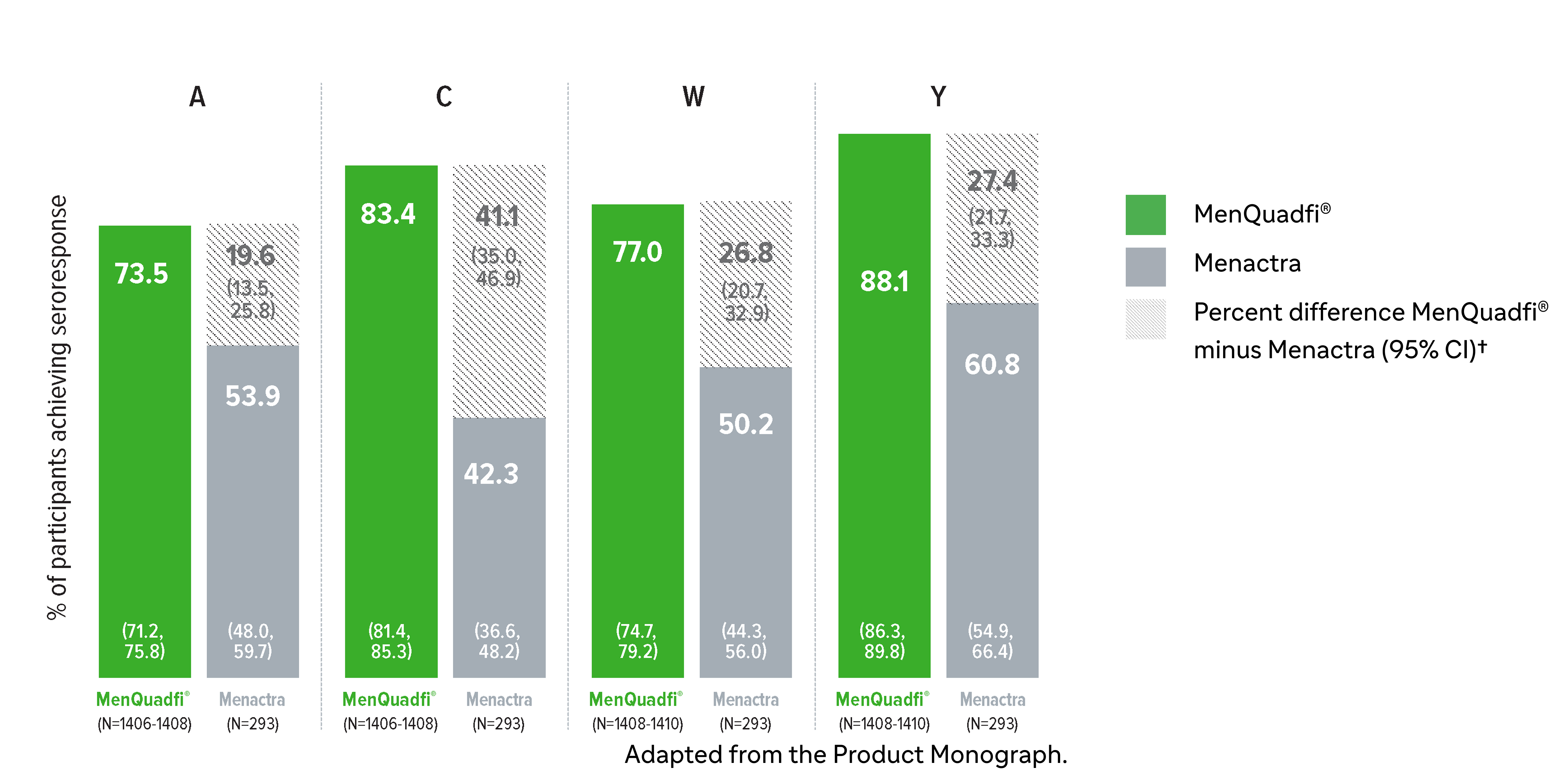
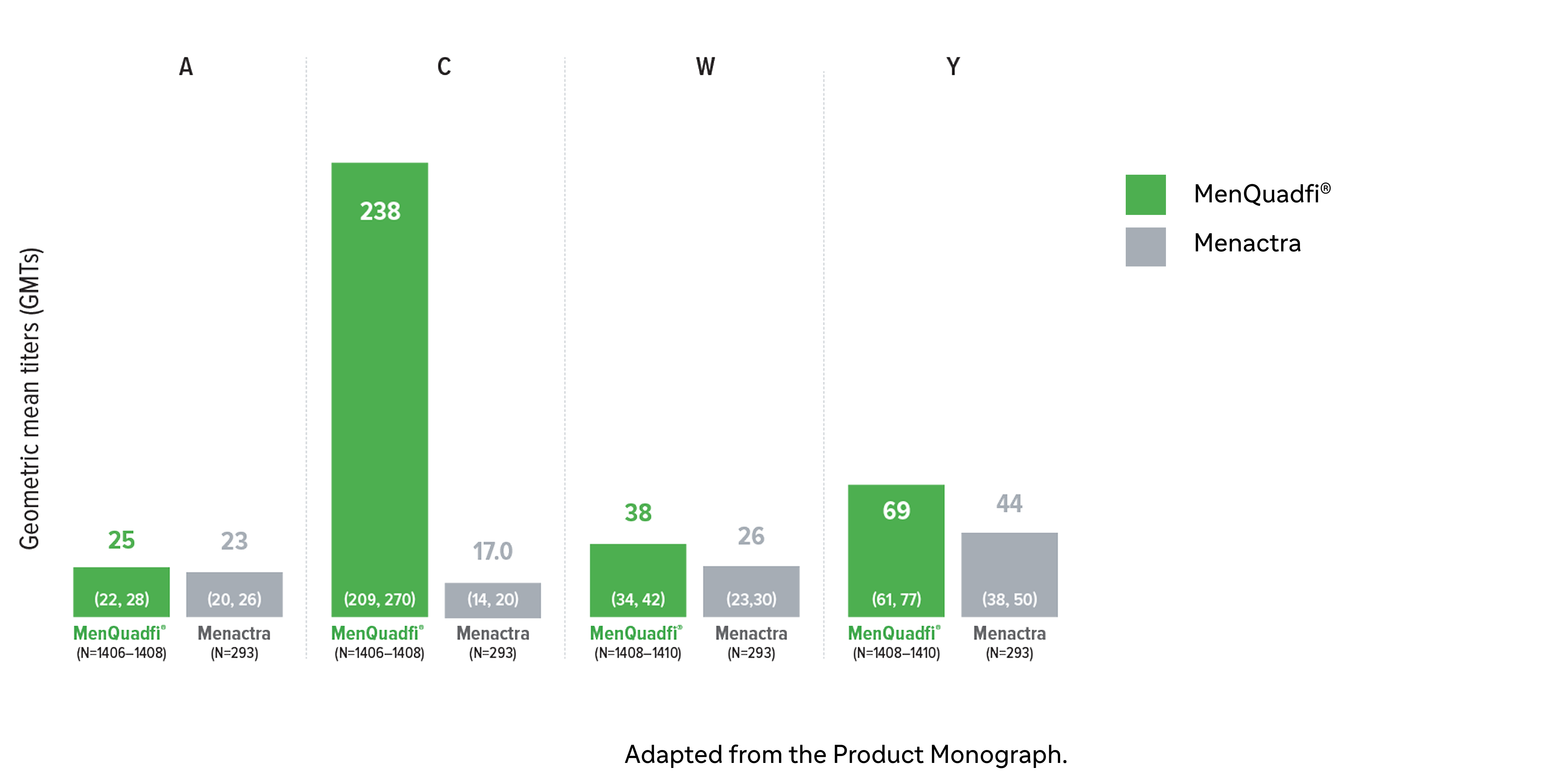
Non-inferiority of immune response was demonstrated for MenQuadfi® vs Menactra for all serogroups (MET43 study)1*
MenQuadfi® vs Menactra1
Percentage of participants achieving seroprotection (95% CI)
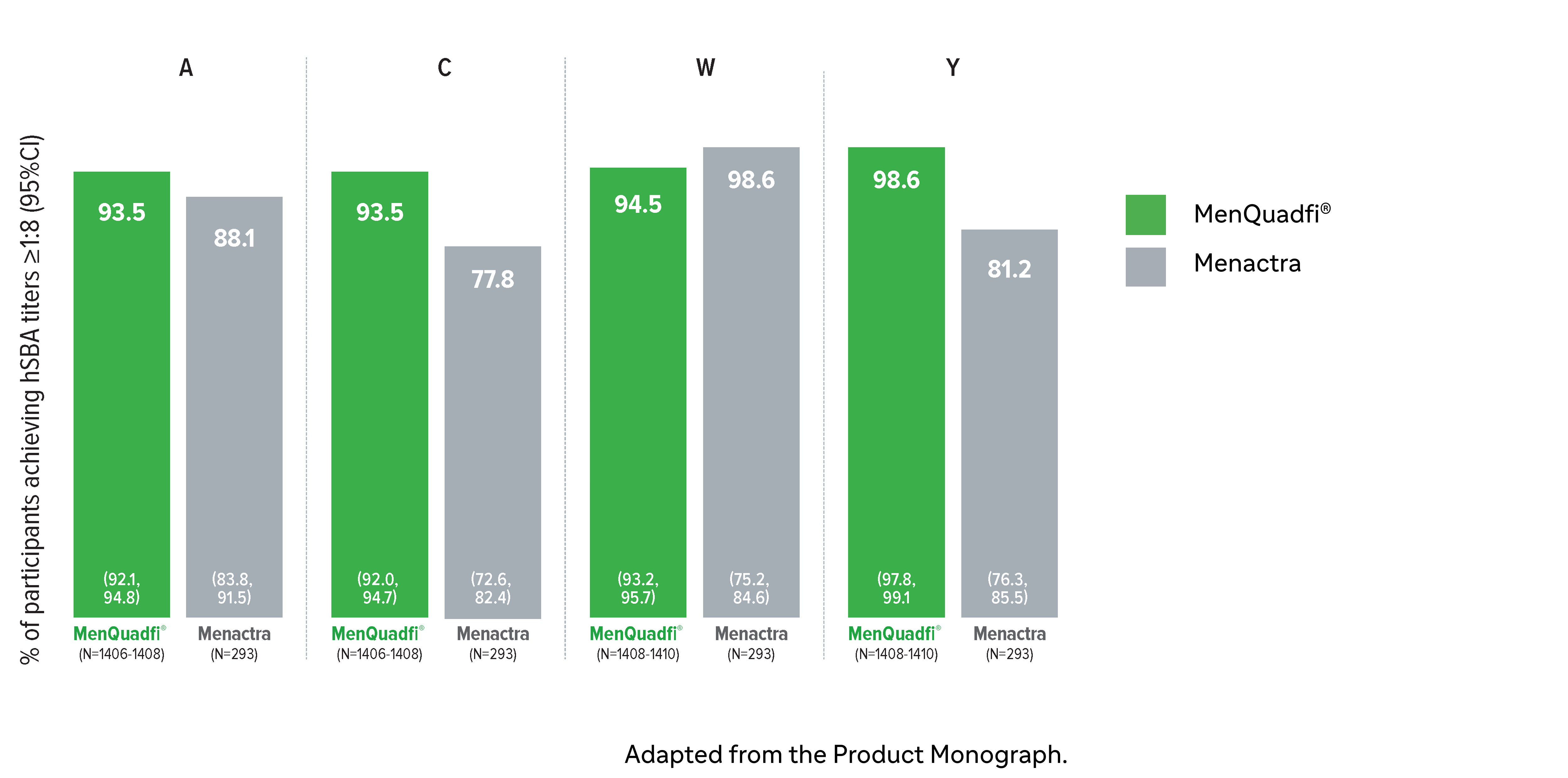
Percentage of participants achieving seroprotection (95% CI)
Geometric mean titers (GMTs)
CI = confidence interval; hSBA = serum bactericidal assay using human complement. N = number of subjects in the per protocol analysis set with valid serology results.
* Randomized, multicentre, active-controlled, modified double-blind study comparing MenQuadfi® (N=1406–1410) to Menactra (N=293) in participants 18–55 years of age.1
† Seroresponse rate (primary endpoint) for each serogroup: the proportion of participants with an hSBA pre-vaccination titer <1:8 who achieved a post-vaccination titer ≥1:16, or pre-vaccination titer ≥1:8 who achieved a post-vaccination titer at least 4-fold greater than the pre-vaccination titer. The overall non-inferiority would be demonstrated if the lower limit of the 2-sided 95% CI is >-10% for all four serogroups.1
Immunogenicity in older adults (≥56 years)
Non-inferiority of immune response was demonstrated for MenQuadfi® vs Menomune for all serogroups (MET49 study)1*
MenQuadfi® vs Menomune1
Percentage of participants achieving seroprotection (95% CI)
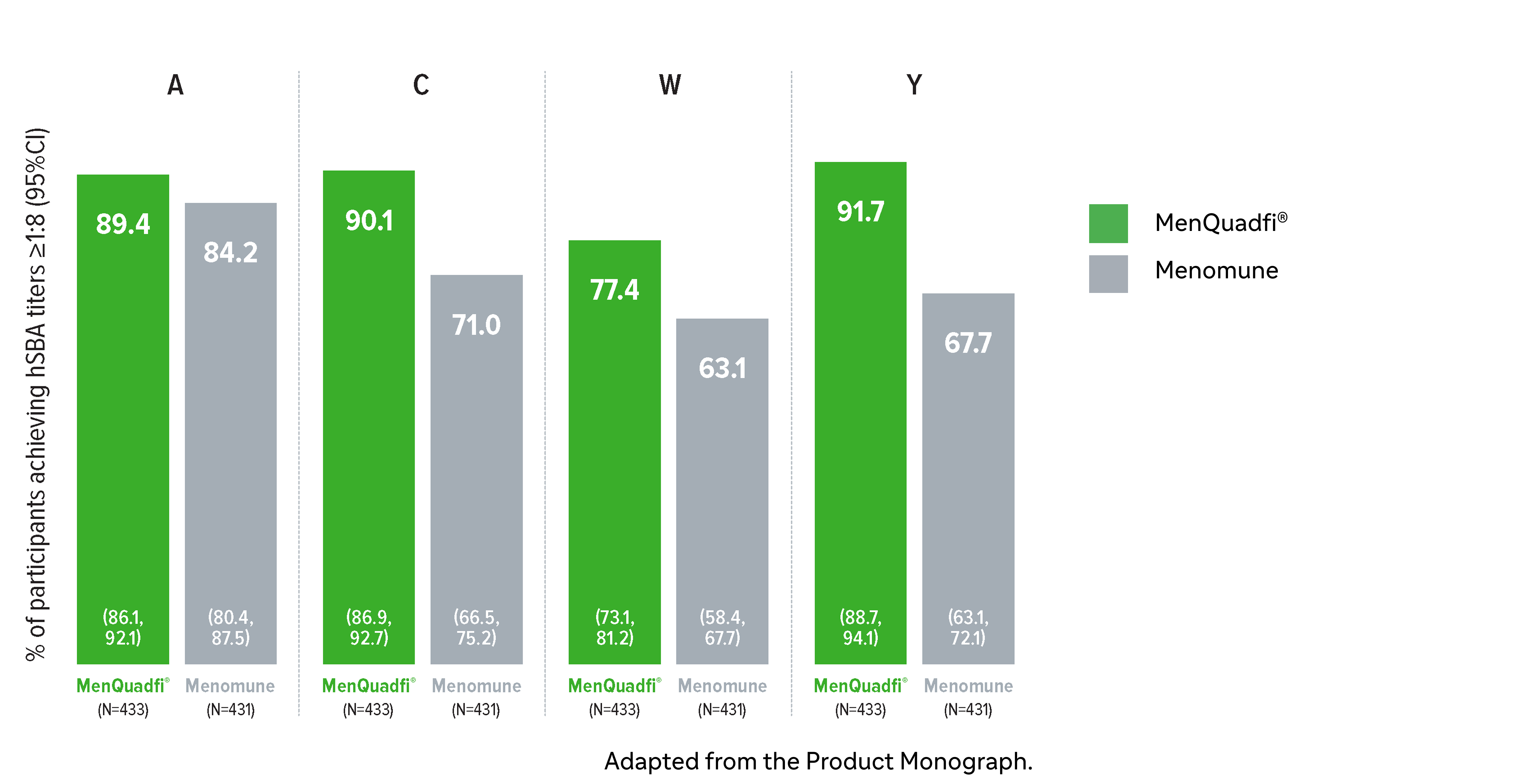
Percentage of participants achieving seroprotection (95% CI)
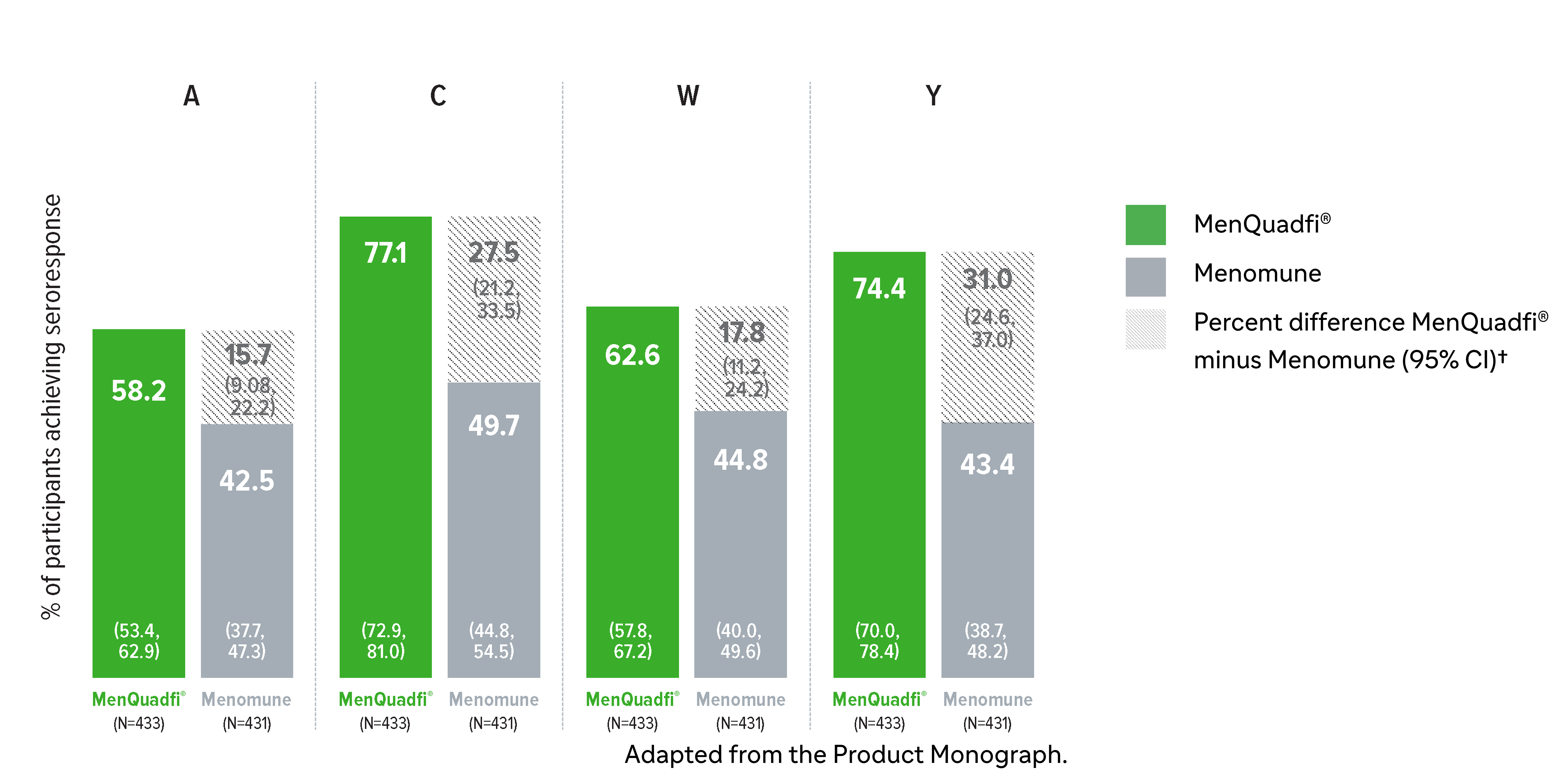
Geometric mean titers (GMTs)
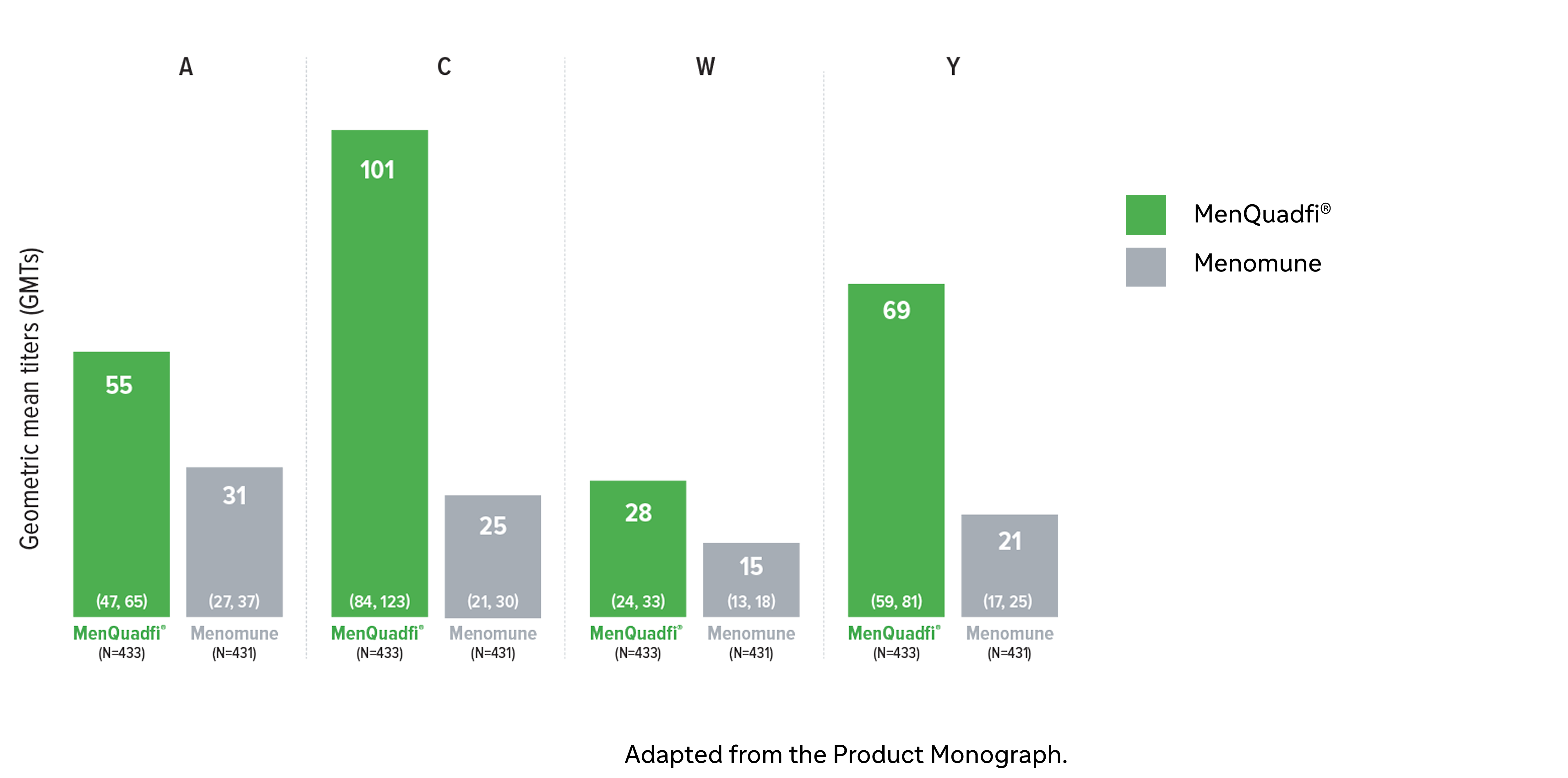
CI = confidence interval; hSBA = serum bactericidal assay using human complement. N = number of subjects in the per protocol analysis set with valid serology results.
* Randomized, multicentre, active-controlled, modified double-blind study comparing MenQuadfi® (N=433) to Menomune (N=431) in participants ≥56 years of age.1
† Seroresponse rate (primary endpoint) for each serogroup: the proportion of participants with an hSBA pre-vaccination titer <1:8 who achieved a post-vaccination titer ≥1:16, or pre-vaccination titer ≥1:8 who achieved a post-vaccination titer at least 4-fold greater than the pre-vaccination titer.1
Dosage and administration
Recommended dosing1
Primary vaccination1
Individuals 12 months of age and older receive a single dose.
Booster vaccination1
A single dose of MenQuadfi® may be administered to adolescents and adults who are at continued risk for meningococcal disease if at least 4 years have elapsed since a prior dose of meningococcal (Groups A, C, W, Y) conjugate vaccine.
There are no data available yet to indicate the need for or timing of a booster dose of MenQuadfi® for individuals who have been primed with MenQuadfi®.
Administration
MenQuadfi® is a ready to use clear, colourless solution. The vaccine should be inspected visually for any particulate matter and/or variation of physical aspect prior to administration. Do not use if the content appears otherwise. Discard any unused portion.
MenQuadfi® should be administered as a single 0.5 mL injection by intramuscular route into the deltoid region or anterolateral thigh, depending on the recipient's age and muscle mass. No data are available to establish safety and immunogenicity of the vaccine using intradermal or subcutaneous routes of administration.
Please see the Product Monograph for complete dosing and administration instructions.
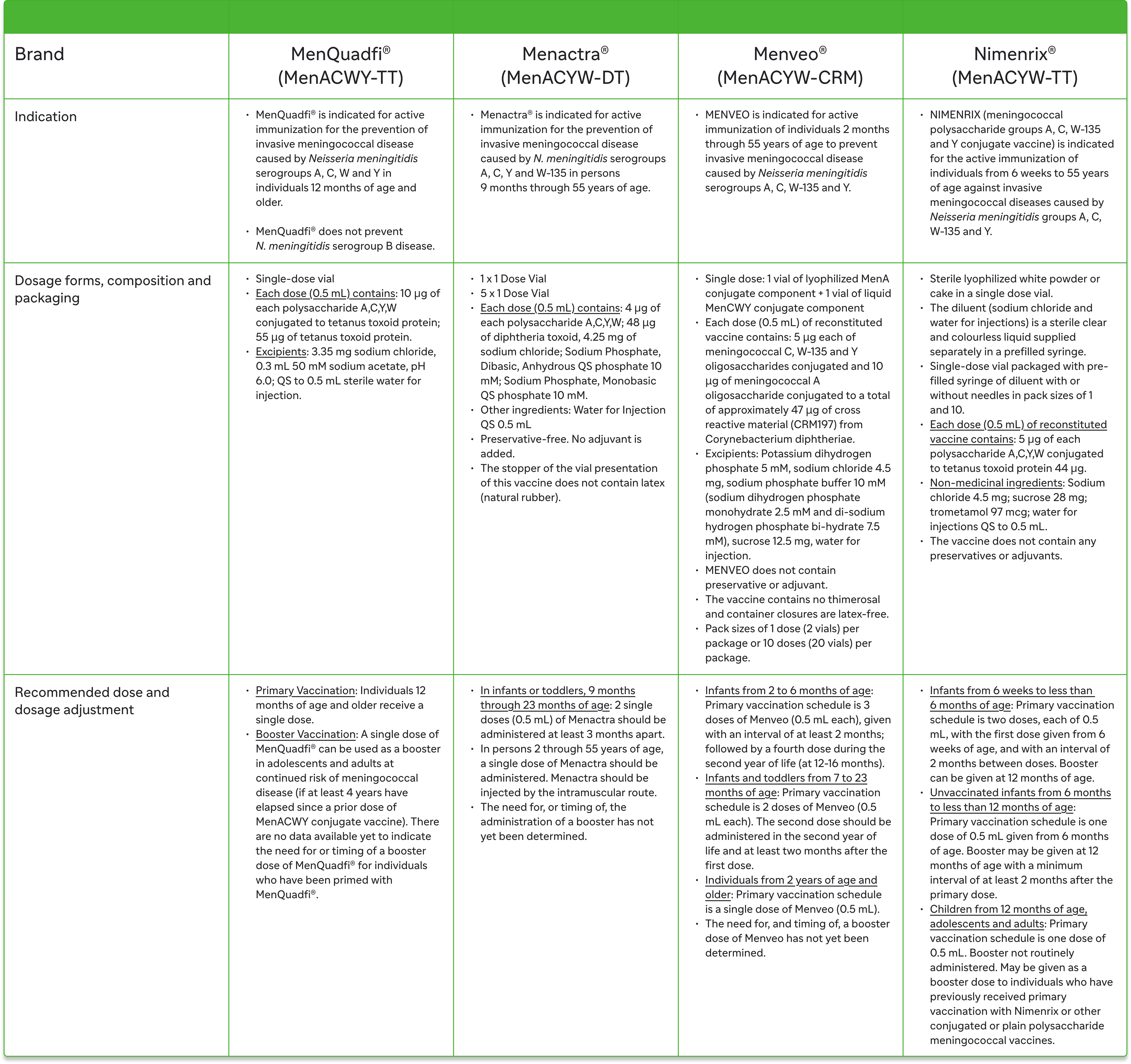
Selected information of MenACWY vaccines in Canada1,2,7-9
Safety information
MenQuadfi® has a well-documented safety profile.1
Overview
The safety of MenQuadfi® in individuals 12 months of age and older is based on 7 pivotal clinical studies in which participants received either MenQuadfi® alone (5327 participants), MenQuadfi® concomitantly with other vaccines (981 participants), the concomitant vaccines without MenQuadfi® (590 participants), or a comparator meningococcal vaccine (2898 participants).
MET51: Toddlers (12–23 months)1
Unsolicited injection-site reactions at the site of MenQuadfi® injection included bruising, haematoma, induration, pruritus, and rash (0.3% each). Unsolicited systemic adverse reactions assessed as vaccine-related by the investigator more than once among recipient of MenQuadfi® and with a frequency of at least 1% (regardless of causal relationship) included diarrhea (MenQuadfi® 7.6%, Nimenrix 5.2%).
SAEs occurred at a rate of 0.7% following MenQuadfi® and at a rate of 0.3% following Nimenrix during the entire study period. No SAEs were determined to be vaccine-related.
MET35 study: Children (2–9 years)1
Unsolicited injection-site reactions at the site of MenQuadfi® injection included bruising (0.4%), induration (0.2%), and warmth (0.2%). Unsolicited systemic adverse reactions assessed as vaccine-related by the investigator more than once among recipients of MenQuadfi® and with a frequency of at least 1% (regardless of causal relationship) included vomiting (MenQuadfi® 2.4%, Menveo 2.2%) and stomach pain (MenQuadfi® 1.4%, Menveo 1.0%).
SAEs occurred at a rate of 1.4% following MenQuadfi® and at a rate of 0.6% following Menveo during the entire study period. No SAEs were determined to be vaccine-related.
MET50 and MET43 studies: Adolescents (10–17 years)1
The safety of MenQuadfi® in participants 10 years through 17 years of age was evaluated in two randomized, controlled clinical trials (MET43 and MET50).
Unsolicited injection-site reactions at the site of MenQuadfi® injection with a frequency of at least 0.1% in either study MET50 when MenQuadfi® was given alone or MET43 included pruritus (0.6% and 0.7%), rash (0.2% and 0.2%), warmth (0.8% and 0.5%), bruising (0.2% and <0.1%) and induration (0.0% and 0.2%). There were no unsolicited systemic adverse reactions assessed as vaccine-related by the investigator more than once among recipients of MenQuadfi® and with a frequency of at least 1% (regardless of causal relationship).
SAEs occurred at a rate of 0.3%–0.8% following MenQuadfi® alone and at a rate of 0.8% following Menveo in study (MET50) and 0.9% following Menactra in Study MET43 during the entire study period. No SAEs were determined to be vaccine-related. A few participants experienced dizziness or syncope within 30 minutes following vaccination (MenQuadfi® 0.2% [dizziness], Menveo 0.2% [syncope], Menactra 0.0%). These events were non-serious and spontaneously resolved on the same day.
MET43 study: Adults (18–55 years)1
Unsolicited injection-site reactions at the site of MenQuadfi® injection with a frequency of at least 0.1% included pruritus (0.8%), warmth (0.3%), and mass (0.1%). There were no unsolicited systemic adverse reactions assessed as vaccine-related by the investigator more than once among recipients of MenQuadfi® and with a frequency of at least 1% (regardless of causal relationship).
A few participants experienced dizziness within 30 minutes following vaccination (MenQuadfi® 0.3%, Menactra 0.3%). These events were non-serious and spontaneously resolved on the same day.
SAEs occurred at a rate of 1.6% following MenQuadfi® and at a rate of 0.6% following Menactra during the entire study period. No SAEs were determined to be vaccine-related.
MET49 study: Older adults (≥56 years)1
Unsolicited injection-site reactions at the site of MenQuadfi® injection included pruritus (1.8%), warmth (0.2%) and ecchymosis (0.2%). There were no unsolicited systemic adverse reactions assessed as vaccine-related by the investigator more than once among recipients of MenQuadfi® and with a frequency of at least 1% (regardless of causal relationship).
SAEs occurred at a rate of 3.3% following MenQuadfi® and at a rate of 3.3% following Menomune during the entire study period. No SAEs were determined to be vaccine-related.
Please refer to the Product Monograph for complete safety profile incidence and adverse reactions in each clinical trial.
Clinical use:
Pediatrics (≥12 months of age): Based on the data submitted and reviewed by Health Canada, the safety and immunogenicity of MenQuadfi® in pediatrics has been established; therefore, Health Canada has authorized an indication for pediatric use.
Geriatrics (≥65 years of age): Based on the data submitted and reviewed by Health Canada, the safety and immunogenicity of MenQuadfi® in geriatrics has been established; therefore, Health Canada has authorized an indication for geriatric use.
Contraindications:
MenQuadfi® is contraindicated in anyone with a known systemic hypersensitivity reaction to any component of MenQuadfi®, including tetanus toxoid, or after a previous administration of the vaccine.
Relevant warnings & precautions:
MenQuadfi® can only protect against N. meningitidis A, C, W and Y serogroups and will not protect against any other microorganisms. It does not prevent N. meningitidis serogroup B disease.
Vaccination with MenQuadfi® should be postponed in individuals suffering from an acute severe febrile illness.
MenQuadfi® should not be administered to subjects with a known history of Guillain-Barré syndrome, unless the potential benefits outweigh the risks of administration.
MenQuadfi® should be given with caution in individuals with thrombocytopenia, any coagulation disorder or to individuals receiving anticoagulant therapy, and only if the potential benefit clearly outweighs the risk of administration.
Appropriate observation and medical treatment should always be readily available in case of an anaphylactic event following the administration of the vaccine.
As with any vaccine, vaccination with MenQuadfi® may not protect all vaccine recipients.
Syncope can occur following or even before any vaccination.
Some individuals with altered immunocompetence, including some individuals receiving immunosuppressant therapy, may have reduced immune responses to MenQuadfi®.
Persons with certain complement deficiencies and persons receiving treatment that inhibits terminal complement activation are at increased risk for invasive disease caused by N. meningitidis, even if they develop antibodies following vaccination with MenQuadfi®.
Immunization with MenQuadfi® vaccine does not substitute for routine tetanus immunization.
MenQuadfi® may temporarily affect the ability to drive or use machines.
MenQuadfi® should be used during pregnancy only if the potential benefits to the mother outweigh the potential risks, including those to the fetus.
MenQuadfi® should be used during breastfeeding only if the potential benefits to the mother outweigh the potential risks, including those to the breastfed child.
Safety and immunogenicity of MenQuadfi® has not been established in individuals less than 12 months of age.
For more information:
Please consult the Product Monograph for important information relating to adverse reactions, drug interactions, and dosing information which have not been discussed in this piece. The Product Monograph is available through our medical department. Call us at 1-800-265-7927.
- MenQuadfi® Product Monograph. Sanofi Pasteur Limited. October 30, 2024.
- Data on File. December 18, 2024.
- Zambrano B, et al. Quadrivalent meningococcal tetanus toxoid-conjugate booster vaccination in adolescents and adults: phase III randomized study. Pediatr Res. 2023;94(3):1035-1043.
- Piazza F, et al. Immunogenicity and safety of an investigational quadrivalent meningococcal conjugate vaccine administered as a booster dose in children vaccinated against meningococcal disease 3 years earlier as toddlers: A Phase III, open-label, multicenter study. Hum Vaccin Immunother. 2022;18(1):1-10.
- Robertson C, et al. Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered as a booster to adults aged ≥59 years: A phase III randomized study. Hum Vaccin Immunother. 2023;19(1):2160600.
- Bröker M, et al. Factors contributing to the immunogenicity of meningococcal conjugate vaccines. Hum Vaccin Immunother. 2016;12(7):1808-1824.
- Menactra PM. Sanofi Pasteur Limited. November 28, 2017.
- Menveo PM. GlaxoSmithKline. June 30, 2020.
- Nimenrix PM. Pfizer. November 28, 2023.
MAT-CA-2401580E-1.0 - 05/2025
MenQuadfi® is a trademark of Sanofi Pasteur. Sanofi Pasteur, 1755 Steeles Avenue West, Toronto, Ontario M2R 3T4
© 2025 Sanofi Pasteur Limited. All rights reserved.





.jpg/jcr:content/image%20(1).jpg)
.jpg/jcr:content/image%20(2).jpg)
.jpg/jcr:content/image%20(3).jpg)