Beyfortus has the power to help reduce the chaos of the RSV season
Sign up to receive the latest information about Beyfortus |
Sign Up |
Profile
Beyfortus is indicated for the prevention of RSV lower respiratory tract disease (LRTD) in:
i. Neonates and infants during their first RSV season.
ii. Children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season (see SmPC).
Beyfortus® (nirsevimab) is a long-acting monoclonal antibody that offers protection against RSV LRTD for at least five months.1
Administration of antibodies via intramuscular injection doesn't rely on an infant’s developing immune system1,2
Beyfortus is a long-acting antibody – it has been modified to have an extended half-life, so that a single dose affords at least 5 months of protection**1
The antibody targets the prefusion conformation of the RSV F protein to inhibit membrane fusion, an essential step in viral entry – this neutralises the virus and blocks cell-to-cell fusion1
**Based on clinical and pharmacokinetic data.1
Safety Profile
The safety profile of nirsevimab has been studied in a broad population, including healthy pre-term/term babies and those at higher risk for severe RSV lower respiratory tract infections (LRTIs).1
Beyfortus was studied across three pivotal studies that enrolled >3800 infants 1, 3-5
- Phase 2b study: 1453 healthy, pre-term infants randomised 2:1 to receive Beyfortus followed by placebo, or palivizumab.
- Phase 3 MELODY (primary cohort) study: 1490 healthy, term and late pre-term infants randomised 2:1 to receive Beyfortus followed by placebo, or palivizumab.
- Phase 2/3 MEDLEY study:925 infants born very or moderately pre-term, or infants with congenital heart disease (CHD) or chronic lung disease (CLD) randomised 2:1 to receive Beyfortus followed by placebo, or palivizumab.
The most frequent adverse reaction was rash (0.7%) occurring within 14 days post dose. The majority of cases were mild to moderate in intensity. Additionally, pyrexia and injection site reactions were reported at a rate of 0.5% and 0.3% within 7 days post dose, respectively. Injection site reactions were non-serious.1 For full adverse events information please refer to the Beyfortus Summary of Product Characteristics.
In a post marketing setting serious hypersensitivity reactions have been reported following Beyfortus administration. Anaphylaxis has been observed with human immunoglobulin G1 (IgG1) monoclonal antibodies. If signs and symptoms of anaphylaxis or other clinically significant hypersensitivity reaction occur, immediately discontinue administration and initiate appropriate medicinal products and/or supportive therapy.1
Adverse reactions reported in 2,966 term and pre-term infants (gestational age [GA] ≥29 weeks) who received Beyfortus in two placebo‑controlled clinical trials and in post-marketing setting.1
| System organ class | Preferred term | Frequency |
| Immune system disorders | Hypersensitivity† | Not known |
| Skin and subcutaneous tissue disorders | Rash* | Uncommon |
| General disorders and administrations site conditions | Injection site reaction** | Uncommon |
| General disorders and administrations site conditions | Pyrexia | Uncommon |
Adapted from Beyfortus. IE Summary of Product Characteristics. September 2024
†Adverse reaction from spontaneous reporting
*Rash was defined by the following grouped preferred terms: rash, rash maculo-papular, rash macular.1
**Injection site reaction was defined by the following grouped preferred terms: injection site reaction, injection site pain, injection site induration, injection site edema, injection site swelling.1
‘Uncommon’ defined as ≥1/1,000 to <1/ 100.1
In the MEDLEY study of infants at higher risk of severe RSV disease, the safety profile of Beyfortus was similar vs. palivizumab.†,1,3
†Safety was evaluated in MEDLEY in 918 infants at higher risk for severe RSV disease, including 196 extremely pre-term infants (GA <29 weeks) and 306 infants with chronic lung disease of prematurity, or hemodynamically significant congenital heart disease entering their first RSV season, who received nirsevimab (614) or palivizumab (304)1
Practical considerations
How should Beyfortus be stored?1
-
Store in a refrigerator (2–8°C)
-
Do not freeze
-
Do not shake or expose to direct heat
-
Keep the pre-filled syringe in the outer carton in order to protect from light
What is the shelf life of Beyfortus?1
3 years
Beyfortus may be kept at room temperature (20‑25°C) when protected from light for a maximum of 8 hours. After this time, the syringe must be discarded
What pack sizes are available in the Republic of Ireland?1
-
Pack of 1 pre-filled syringe without a needle
-
Pack of 1 pre-filled syringe with two separate needles of different sizes
Understanding the chaos of RSV season
All infants are at risk from respiratory syncytial virus (RSV) lower respiratory tract disease (LRTD),*,7-13 but we cannot predict who will be affected.14
RSV LRTD is a leading cause of hospitalisation in infants, regardless of health status, gestational age at birth, or month of birth.**,7-13,15-18
Among those hospitalised
due to RSV:
This puts a strain on healthcare services...
of children were born healthy and at term (across different countries)*,7-13
of infants were born during the season, and ~50% before it began†,7,16,17
of healthcare professionals (HCPs) from Europe reported moderate to extreme disruption to healthcare systems during peak RSV season‡,19
*Percentage of children born healthy and/or full term among children hospitalised due to RSV in different retrospective analyses: France (2010-2018; in children <5 years of age): 87% healthy and 90% full term;7 Spain (2004-2012; in infants): 98% healthy and full term;8 Japan (January 2017-December 2018; in children ≤2 years of age): 90% healthy and full term;9 Germany (2015-2018; in infants): 90% healthy and 83% full term;10 China (2007-2015, in children 28 days-13 years of age): 88% healthy and full term (median age 1.4 years);11 UK (Scotland 2000-2011; in children ≤2 years of age): 93% healthy and 82% full term;13 US (population-based surveillance 2014-2015; in infants ≤11 months): 72% healthy and full term.12
**From retrospective analyses conducted in France (45,225 RSV-associated hospitalisations per season between 2010 and 2018)7 and US (243,834) RSV bronchiolitis, 38,064 RSV pneumonia and 15,786 other RSV discharges of infants <1 year of age between 1997 and 1999).15
†Based on an English study where 51% (n=10,328/20,359) of hospital admissions due to RSV occurred in infants born before the season (April to October);17 a Spanish study where 54% (n=340/631) of infants hospitalised with RSV were born before the season (April through October);16 and a French study where 47% (n=85,292/181 ,758) of RSV-hospitalised infants were born before the season (April to October).7
‡From a cross-sectional survey of 380 HCPs in 20 European countries conducted between August 2021 and January 2022.19
HCP, healthcare professional; LRTD, lower respiratory tract disease; LRTI, lower respiratory tract infection; RSV, respiratory syncytial virus.
Dosing & Administration
When and how to use Beyfortus
Beyfortus should be administered from birth for infants born during the RSV season. For others born outside the season Beyfortus should be administered ideally prior to RSV season.1
Beyfortus is available in a 50 mg and a 100 mg pre-filled syringe based on an infant’s weight at the time of receiving Beyfortus.*,1 Beyfortus is given as an intramuscular injection ONLY, preferably in the anterolateral aspect of the thigh.**,1
Different injection sites should be used
*The recommended dose is a single dose of 50 mg administered intramuscularly for infants with body weight <5 kg and a single dose of 100 mg administered intramuscularly for infants with body weight ≥5 kg.1
Please consult the Summary of Product Characteristics for complete information of full dosing and administration information.
**The gluteal muscle should not be used routinely as an injection site because of the risk of damage to the sciatic nerve.1
Beyfortus is a long-acting antibody against RSV LRTD1
Beyfortus confers protection through the delivery of antibodies, rather than depending on the maturation of infants immune system to produce their own antibodies.1,2
Based on clinical and pharmacokinetic data, the duration of protection afforded by Beyfortus is at least 5 months.1
Beyfortus Mode of Action1
Beyfortus is a recombinant neutralising human IgG1ĸ long-acting monoclonal antibody (mAb) to the prefusion conformation of the RSV F protein which has been modified with a triple amino acid substitution (YTE) in the Fc region to extend serum half-life.1
Beyfortus clinical data
HARMONIE study: Impact on Incidence of RSV Hospitalization
A Phase 3b open-label, multi-centre, parallel 2-arm study performed comparing nirsevimab to no preventative RSV intervention (standard of care) in preventing hospitalisations due to RSV in infants 22
The Beyfortus development programme was conducted across a broad infant population.1,3-5
This included healthy infants born at term, premature infants, and those with congenital heart disease and chronic lung disease of prematurity.1,3-5
- Phase 2b study: 1453 healthy pre-term infants
- Phase 3 MELODY (primary cohort) study: 1490 healthy, term and late pre-term infants
- Phase 2/3 MEDLEY study: 925 infants born very or moderately pre-term, or infants with congenital heart disease (CHD) or chronic lung disease (CLD)
>3,800 infants3-5
3 pivotal studies3-5
In 31 countries3-5
FAQs
Got a question about Beyfortus?
We are committed to answering your questions about Beyfortus. If you don’t find an answer to your question below, or would like more information, please get in touch.
Beyfortus is indicated for the prevention of RSV lower respiratory tract disease (LRTD) in:
i. Neonates and infants during their first RSV season.
ii. Children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season (see SmPC).1
Beyfortus should be used in accordance with official recommendations.1
- Beyfortus provides antibodies via intramuscular injection offering protection against RSV LRTD which doesn’t rely on an infant’s maturing immune system1,20,21
- Beyfortus is a long-acting monoclonal antibody that has been modified to extend its half-life, affording at least 5 months of protection*,1
- Beyfortus is an antibody targeted to the prefusion conformation of the RSV F protein; it inhibits the essential membrane fusion step in the viral entry process, neutralising the virus and blocking cell-to-cell fusion1
- Find out more about the Beyfortus mechanism of action
*Based on clinical and pharmacokinetic data.1
- Beyfortus has been studied across a broad infant population in the phase 2b, MELODY and MEDLEY studies (healthy pre-term infants, healthy term infants and infants with underlying conditions, born during or before respiratory syncytial virus season)1,3-5
-
Beyfortus is indicated for the prevention of Respiratory Syncytial Virus (RSV) lower respiratory tract disease in:1
i. Neonates and infants during their first RSV season.
ii. Children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
Beyfortus should be used in accordance with official recommendations.1 - Find out more about the infant populations included in the clinical trials
- Based on clinical and pharmacokinetic data, Beyfortus offers protection against RSV LRTD for at least 5 months.1
- Beyfortus was studied across three pivotal studies that enrolled >3,800 infants:1,3-5
- Phase 2b study: 1,453 healthy, pre-term infants randomised 2:1 to Beyfortus followed by placebo, or palivizumab.
- Phase 3 MELODY (primary cohort) study: 1,490 healthy, term and late pre-term infants randomised 2:1 to Beyfortus followed by placebo, or palivizumab.
- Phase 2/3 MEDLEY study: 925 infants born very or moderately pre-term, or infants with haemodynamically significant congenital heart disese (CHD) or chronic lung disease (CLD) of prematurity randomised 2:1 to Beyfortus followed by placebo, or palivizumab.
- The most frequent adverse reaction was rash (0.7%) occurring within 14 days post dose. The majority of cases were mild to moderate in intensity. Additionally, pyrexia and injection site reactions were reported at a rate of 0.5% and 0.3% within 7 days post dose, respectively. Injection site reactions were non-serious.*,1
- In the MEDLEY study (infants at higher risk of severe RSV disease), the safety profile of Beyfortus was similar vs. palivizumab1,3
- In a post marketing setting serious hypersensitivity reactions have been reported following Beyfortus administration. Anaphylaxis has been observed with human immunoglobulin G1 (IgG1) monoclonal antibodies. If signs and symptoms of a clinically significant hypersensitivity reaction or anaphylaxis occur, immediately discontinue administration and initiate appropriate medicinal products and/or supportive therapy.1
- Find out more about the Beyfortus safety profile
*Rash was defined by the following grouped preferred terms: rash, rash maculo‐papular, rash macular. Injection site reaction was defined by the following grouped preferred terms: injection site reaction, injection site pain, injection site induration, injection site oedema, injection site swelling.1
- For infants during their first RSV season: Beyfortus should be administered from birth for infants born during the RSV season.1
- For others born outside of the season Beyfortus should be administered ideally prior to the RSV season.1
- For children who remain vulnerable to severe RSV disease through their second season: Beyfortus should be administered ideally prior to the start of the second RSV season.1
- Beyfortus can be given concomitantly with other childhood vaccines.1
- Beyfortus should not be mixed with any vaccine in the same syringe or vial. When administered concomitantly with injectable vaccines, they should be given with separate syringes and at different injection sites.1
- Click here for guidance on when to administer Beyfortus
- Beyfortus involves a single administration of either 50 mg or 100 mg, depending on body weight, via a ready-to-use pre-filled syringe1
- Beyfortus is given as an intramuscular injection ONLY, preferably in the anterolateral aspect of the thigh*,1
- Learn more about how to administer Beyfortus
*The gluteal muscle should not be used routinely as an injection site because of the risk of damage to the sciatic nerve.1
-
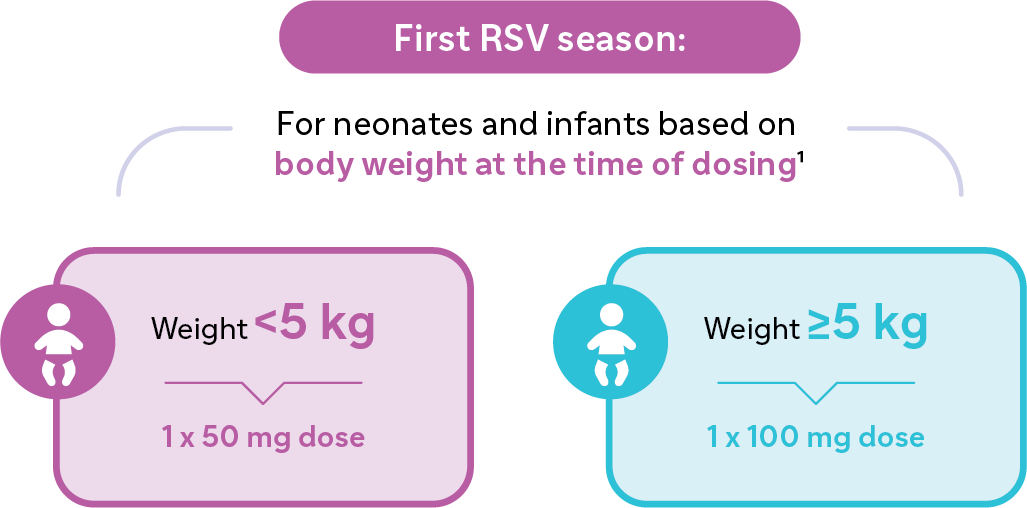
First RSV Season:
- For neonates and infants based on body weight at the time of dosing1
- Infants that weigh <5kg: a single intramuscular injection of 50mg
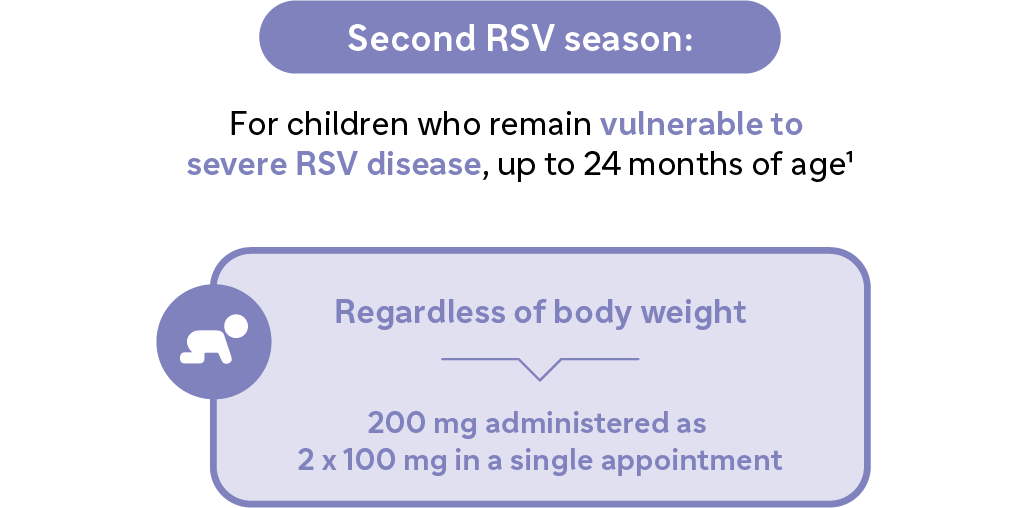
- Infants that weigh ≥ 5kg: a single intramuscular injection of 100mgSecond RSV Season:
- For children who remain vulnerable to severe RSV disease, up to 24 months of age1
- Regardless of body weight: 200mg given as two intramuscular injections (2 x 100mg), administered in clear separate injection sites and ideally prior to the start of the second RSV season.1 - Dosing in infants with a body weight from 1.0 kg to <1.6 kg is based on extrapolation, no clinical data are available. Exposure in infants <1 kg is anticipated to yield higher exposures than in those weighing more. The benefits and risks of nirsevimab use in infants <1 kg should be carefully considered.1
- Click here for more information on Beyfortus dosing
- We've designed some tools and resources to support you on how to discuss RSV and Beyfortus with parents.
- Click here to see tools and resources available
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspect adverse reactions.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie. Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 4013 5600.
Alternatively, send via email to IEPharmacovigilance@sanofi.com
HCP, healthcare professional; LRTD, lower respiratory tract disease; LRTI, lower respiratory tract infection; RSV, respiratory syncytial virus.
- Beyfortus®. IE Summary of Product Characteristics (SmPC). September 2024
- Heinonen S et al. Immunol Allergy Clin North Am 2019; 39(3):361-376
- Domachowske J et al. N Engl J Med 2022; 386(9): 892-894 & Supplementary Appendix
- Griffin MP et al. N Engl J Med 2020; 383(5): 415-425 & Supplementary Appendix
- Hammitt LL et al. N Engl J Med 2022; 386(9): 837-846 & Supplementary Appendix
- Muller WJ et al. N Engl J Med 2023; 388(16): 1533-1534
- Demont C et al. BMC Infect Dis 2021; 21(7): 730
- Sanchez-Luna M et al. Curr Med Res Opin 2016; 32(4); 693-698
- Kobayashi Y et al. Ped Intl 2021; 64(1); e14957
- Yu et al. Emerg Infect Dis 2019; 25(6): 1127-1135
- Hartmann K et al. J Infect Dis 2022; 226(3): 386-395
- Arriola C et al J Pediatric Infect Dis Soc 2020; 9(5): 587-595
- Thwaites R et al. Eur J Pediatr 2020; 179(5): 791-799
- Bianchini S et al. Microorganisms 2020; 8(12): 2048
- Leader S and Kohlhase K. Pediatr Infect Dis J 2002; 21(7): 629-632
- Mira-Iglesias A et al. Influenza Other Respir Viruses 2022
- Reeves RM et al. J Infect 2019; 78(6): 468-475
- Gantenburg JR et al. J Infect Dis 2022; 226(Suppl 2): S164-S174
- European Health Management Association. The Health System Burden of Respiratory Syncytial Virus (RSV) Available at https://www.vaccinestogether.org/the_health_system_burden_of_rsv_in_europe_ehma_s_white_paper_new. Accessed March 2025
- Esposito S et al. Front Immunol 2022; 13:880368
- Slifka MK and Amanna IJ. Plotkin’s Vaccines. Seventh Edition. Chapter 8, Passive Immunisation. Elsevier Inc. 2018
- Drysdale et al. N Engl J Med 2023 Dec 28; 389:2425-2435
MAT-IE-2400241 (v3.0)
Date of Preparation: March 2025