Profile
Suvezen is an HMG-coA reductase inhibitor in combination with other lipid-modifying agents, rosuvastatin and ezetimibe.
Presentations:
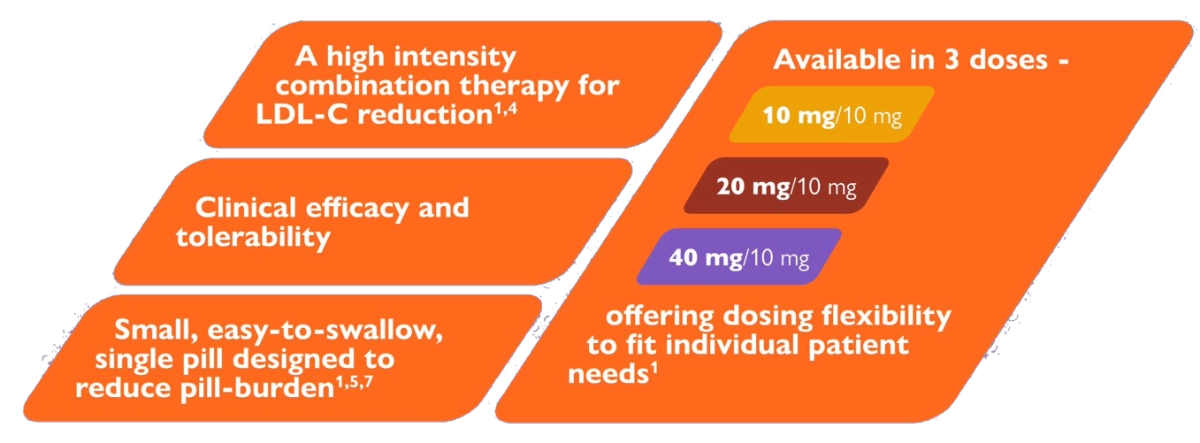
Suvezen 10mg/10mg, 20mg/10mg and 40mg/10mg:
Each film-coated tablet contains 10mg; 20mg or 40mg of rosuvastatin (as rosuvastatin calcium) respectively, and 10mg ezetimibe.
Indication1
Primary Hypercholesterolaemia/Homozygous Familial Hypercholesterolaemia (HoFH)
Suvezen is indicated as adjunct to diet for treatment of primary hypercholesterolaemia (heterozygous familial and non-familial) or homozygous familial hypercholesterolaemia in adult patients
- who are not appropriately controlled with statin alone,
- who are adequately controlled with rosuvastatin and ezetimibe given concurrently at the same dose level as in the fixed combination, but as separate products.
Prevention of Cardiovascular Events
Suvezen is indicated as substitution therapy in adult patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently, at the same dose level as in the fixed dose combination, but as separate products to reduce the risk of cardiovascular events in patients with coronary heart disease (CHD) and a history of acute coronary syndrome (ACS).
Clinical efficacy and tolerability
Clinical efficacy of adding ezetimibe to statin monotherapy has been demonstrated with no worsening of side effects following the addition of ezetimibe.2
Safety and tolerability
|
Organ system |
Adverse events and frequency |
|
Blood and lymphatic system disorders |
Rare: Thrombocytopeniab |
|
Immune system disorders |
Rare: Hypersensitivity reactions including angioedemab |
|
Endocrine disorders |
Common: Diabetes mellitusa,b |
|
Metabolism and nutrition disorders |
Uncommon: Decreased appetitec |
|
Psychiatric disorders |
Not known: Depressionb,e |
|
Nervous system disorders |
Common: Headacheb,d, dizzinessb |
|
Eye disorders |
Unknown: Ocular myastheniab |
|
Vascular disorders |
Uncommon: Hot flushc, hypertensionc |
|
Respiratory, thoracic and mediastinal disorders |
Uncommon: Coughc |
|
Gastrointestinal disorders |
Common: Constipationb, nauseab, abdominal painb,c, diarrhoeac, flatulencec |
|
Hepatobiliary disorders |
Rare: Increased hepatic transaminasesb |
|
Skin and subcutaneous tissue disorders |
Uncommon: Pruritusb,d, rashb,d, urticariab,d |
|
Musculoskeletal and connective tissue disorders |
Common: Myalgiab,d |
|
Renal and urinary disorders |
Very rare: Haematuriab |
|
Reproductive system and breast |
Very rare: Gynaecomastiab |
|
Investigations |
Common: ALT and/or AST increasedd |
|
General disorders and administration site conditions |
Common: Astheniab, fatiguec |
Please refer to the full SmPC for the complete list.1
Adverse reactions have been ranked under headings of frequency using the following convention: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated from the available data).a frequency will depend on the presence or absence of risk factors (fasting blood glucose ≥ 5.6 mmol/l, BMI >30 kg/m2, raised triglycerides, history of hypertension) for rosuvastatin.b Adverse reaction profile for rosuvastatin based on data from clinical studies and extensive post-marketing experience.c Ezetimibe in monotherapy. Adverse reactions were observed in patients treated with ezetimibe (n=2,396) and at a greater incidence than placebo (n=1,159).d Ezetimibe coadministered with a statin. Adverse reactions were observed in patients with ezetimibe co-administered with a statin (n=11,308) and at a greater incidence than statin administered alone (n=9,361).e Additional adverse reactions of ezetimibe, reported in postmarketing experience (with or without statin).
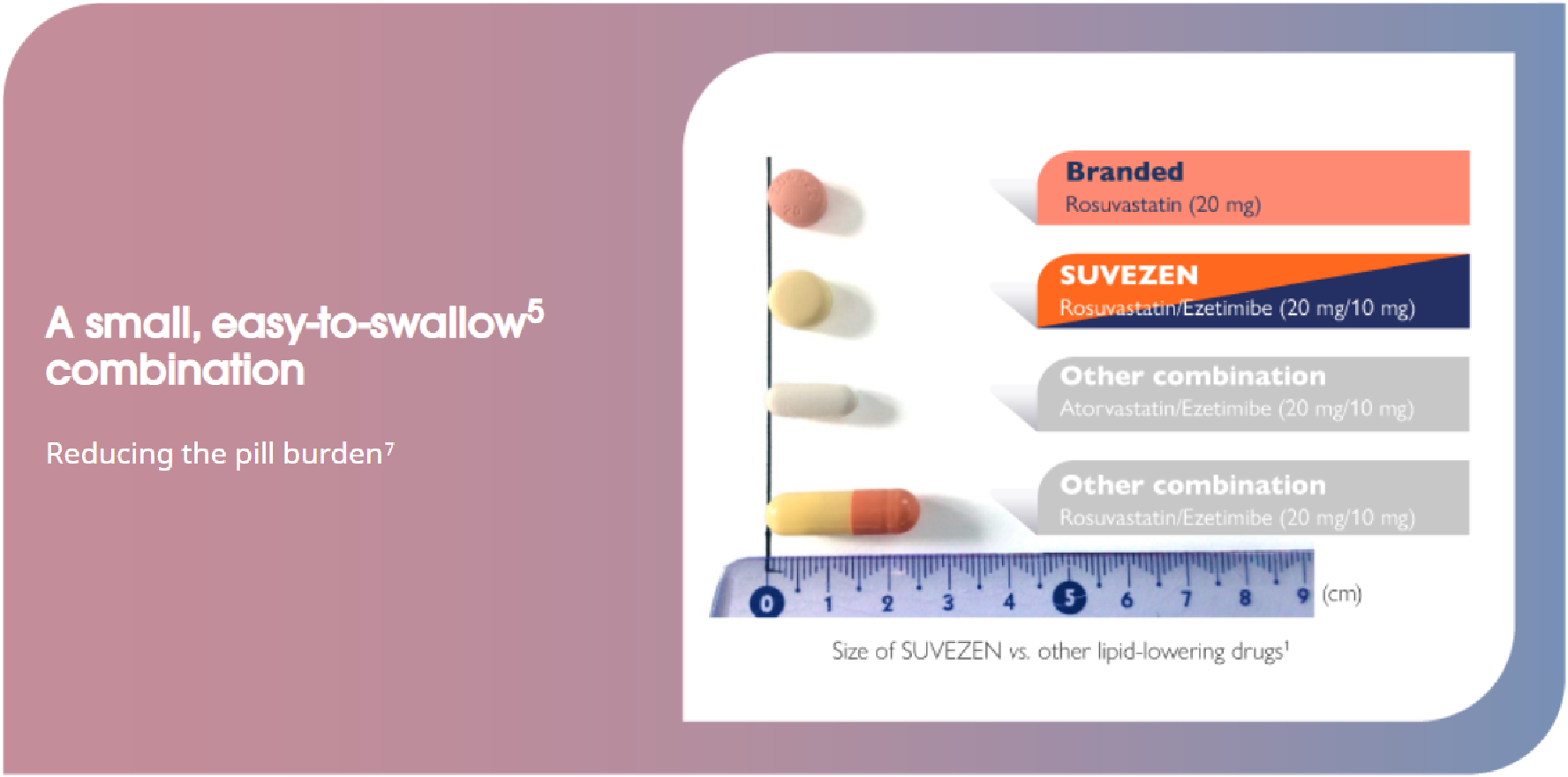
Flexibility of dose range to suit individual patient needs1
To be taken once per day; at the same time; with or without meals; as part of a low-fat dietary regimen.
Mode of Action
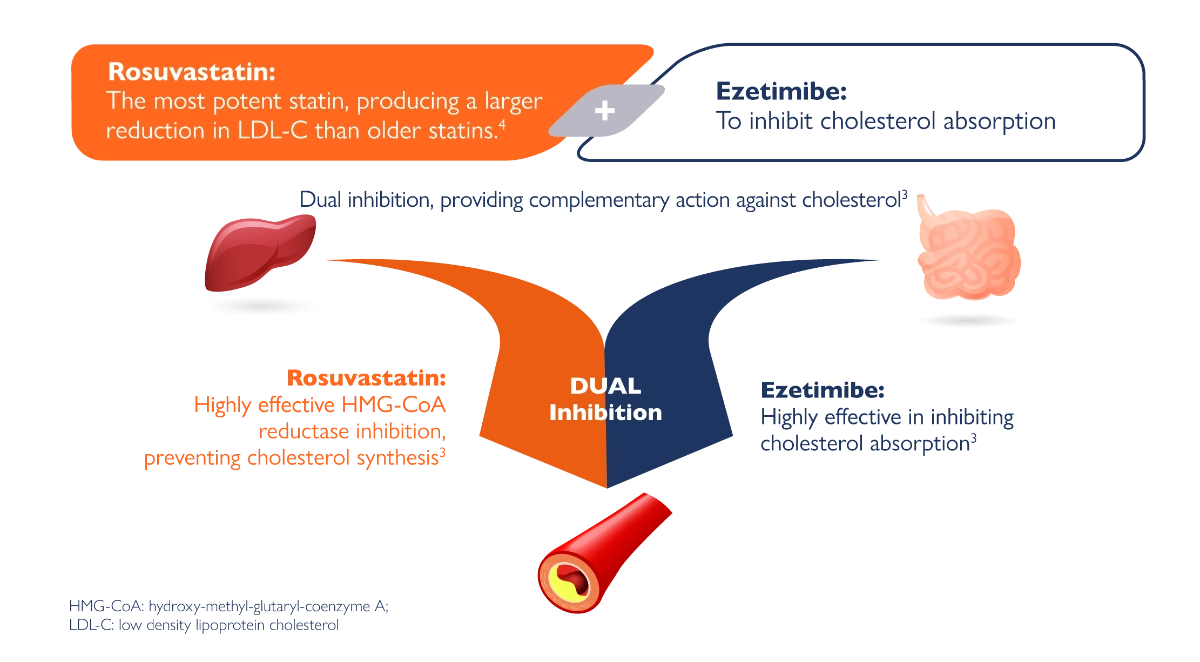
Plasma cholesterol is derived from intestinal absorption and endogenous synthesis. Suvezen contains rosuvastatin and ezetimibe, two lipid-lowering compounds with complementary mechanisms of action. Suvezen reduces elevated total cholesterol (total-C), LDL C, apolipoprotein B (Apo B), triglycerides (TG), and non-high-density lipoprotein cholesterol (non-HDL C), and increases high-density lipoprotein cholesterol (HDL C) through dual inhibition of cholesterol synthesis and absorption.1,5
Suvezen® contains ezetimibe and rosuvastatin, two lipid-lowering compounds with complementary mechanisms of action.
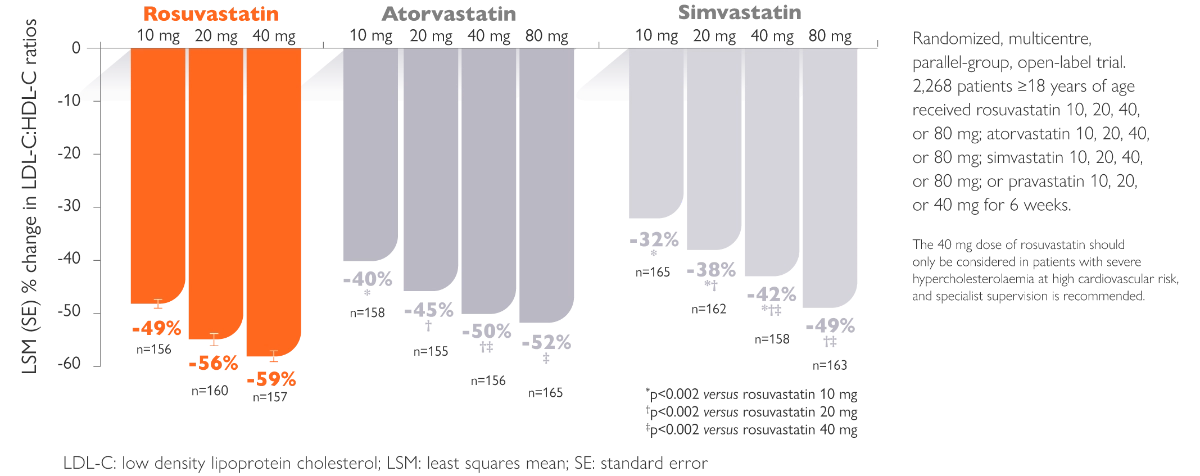
Rosuvastatin produces larger reductions in LDL-C than older statins4
Administration1
The patient should be on and continue, an appropriate lipid-lowering diet, during treatment with Suvezen. Suvezen is not suitable for initial therapy.
When Suvezen is indicated for patients not controlled by statin alone, the dose of Suvezen should be individualized according to the target lipid levels and the patient's response.
When Suvezen is indicated for patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently at the same dose level as in the fixed combination, but as separate product, treatment initiation or dose adjustment if necessary should only be done with the monocomponents and after setting the appropriate doses the switch to the fixed dose combination of the appropriate strength is possible.
Patient should use the strength corresponding to their previous treatment.
The recommended dose is one Suvezen tablet daily. To be administered at any time of the day, with or without food.
The tablet should be swallowed whole with a drink of water. If co-administered with bile acid sequestrant (BAS), administration of Suvezen should occur either ≥2 hours before or ≥4 hours after administration of a BAS.
Patient Profiles
Which of these patients could benefit from treatment with SUVEZEN?
Primary prevention with
medium intensity statin

High CV risk
- 46 years old
-
Diabetic, HbA₁꜀: 7.5%
-
Hypertension, SBP: 155 mmHg
-
LDL-C: 2.6 mmol/L
-
Previous statin-related muscle pain
-
Simvastatin 20 mg
Diabetic with hyperlipidaemia and hypertension
Secondary prevention with
high intensity statin

Very high CV risk
- 55 years old
- MI 6 weeks ago
- LDL-C: 3.3 mmol/L
- Atorvastatin 80 mg at discharge
Recent ACS event

Very high CV risk
- 65 years old
- Established ASCVD, first ACS 18 months ago
- LDL-C: 2.1 mmol/L
- CABG
- Rosuvastatin 10 mg
Non-recent ACS event
ACS = acute coronary syndrome; CABG = coronary artery bypass graft; CV = cardiovascular; HbA1c = haemoglobin A1c; LDL-C = low-density lipoprotein cholesterol; MI = myocardial infarction; SBP = systolic blood pressure.
ESC/EAS guidelines:
Which CV risk category is this patient in?6
High CV risk
- Diabetic with hyperlipidaemia and hypertension
- LDL-C: 2.6 mmol/L
High CV risk
- Markedly elevated single risk factors, in particular TC > 8 mmol/L (>310 mg/dL), LDL-C > 4.9 mmol/L (>190 mg/dL), or BP ≥ 180/110 mmHg
- Patients with FH without other major risk factors
- Patients with DM without target organ damage, with DM duration ≥ 10 years or another additional risk factor
- Moderate CKD (eGFR 30-59 mL/min/1.73 m2)
- Calculated SCORE ≥ 5% and < 10% for 10-year risk of fatal CVD

High CV risk6
- LDL-C goal: < 1.8 mmol/L AND a ≥ 50% reduction from baseline
Next Steps...
Escalate treatment1,6
- Add Suvezen if not appropriately controlled with statin alone
Follow up
- Response to therapy can be assessed 6-8 weeks from therapy initiation
- LDL-C should be assessed whenever available, with a full lipid profile if possible
BP = blood pressure; CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; EAS = European Atherosclerosis Society; ESC = European Society of Cardiology; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; SCORE = systematic coronary risk estimation; TC = total cholesterol.
ESC/EAS guidelines:
Which CV risk category is this patient in?6
Very High CV risk
- MI 6 weeks ago
- LDL-C: 3.3 mmol/L
- Atorvastatin 80 mg at discharge
Recent ACS event
Very High CV risk
- Documented ASCVD, including previous ACS or other documented ASCVD (clinical/imaging)
- DM with target organ damage, or ≥ 3 major risk factors, or early onset of T1DM of long duration (>20 years)
- Severe CKD (eGFR < 30 mL/min/1.73 m2)
- Caculated SCORE ≥ 10% for 10-year risk of fatal CVD
- FH with ASCVD or with another major risk factor

Prior ACS event
=
Very high CV risk6
LDL-C goal: <1.4 mmol/L AND a ≥ 50% reduction from baseline
Next Steps...
Escalate treatment1,4,6
- Switch patient to Rosuvastatin if not at goal with maximum tolerated atorvastatin
- Add ezetimibe if still not at goal with maximum tolerated statin
Follow up6
- Follow up every 4-6 weeks when not at target
- Substitute for Suvezen when the patient has stabilised on the monocomponents
ACS = acute coronary syndrome; ASCVD = atherosclerotic cardiovascular disease; CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; EAS = European Atherosclerosis Society; ESC = European Society of Cardiology; FH = familial hypercholesterolaemia; LDL-C = low- density lipoprotein cholesterol; MI = myocardial infarction; PCSK9i = pro protein convertase subtilisin/kexin type 9 inhibitor; SCORE = systematic coronary risk estimation; T1DM = type 1 diabetes mellitus.
ESC/EAS guidelines:
Which CV risk category is this patient in?6
Very High CV risk
- Established ASCVD, first ACS 18 months ago
- LDL-C: 2.1 mmol/L
- Rosuvastatin 10 mg, CABG
Non-recent ACS event
Very High CV risk
- Documented ASCVD, including previous ACS or other documented ASCVD (clinical/imaging)
- DM with target organ damage, or ≥ 3 major risk factors, or early onset of T1DM of long duration (>20 years)
- Severe CKD (eGFR < 30 mL/min/1.73 m2)
- Calculated SCORE ≥ 10% for 10-year risk of fatal CVD
- FH with ASCVD or with another major risk factor

Prior ACS event
=
Very high CV risk6
LDL-C goal: <1.4 mmo/L AND a ≥ 50% reduction from baseline
Next Steps...
Escalate treatment6
- Add ezetimibe if not at goal with maximum tolerated statins
Follow up6
- Follow up every 4-6 weeks when not at target
- Substitute for Suvezen when the patient has stabilised on the monocomponents
ACS = acute coronary syndrome; ASCVD = atherosclerotic cardiovascular disease; CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; EAS = European Atherosclerosis Society; ESC = European Society of Cardiology; FH = familial hypercholesterolaemia; LDL-C = low- density lipoprotein cholesterol; MI = myocardial infarction; PCSK9i = pro protein convertase subtilisin/kexin type 9 inhibitor; SCORE = systematic coronary risk estimation; T1DM = type 1 diabetes mellitus.
Summary
*LDL C: low density lipoprotein cholesterol;
- SuvezenTM Summary of Product Characteristics. Available from www.medicines.ie last accessed April 2024.
- Ballantyne CM, et al. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). Am J Cardiol. 2007;99(5):673-80.
- Krahenbuhl S, et al. Unmet needs in LDL-C lowering: when statins won’t do! Drugs. 2016;76(12):1175-90.
- Jones PH et al. Effects of rosuvastatin versus atorvastatin, simvastatin and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin Ther. 2004;26 (9):1388-99.
- Schiele JT, Quinzler R, Klimm HD et al. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013; 69:937-948.
- Mach F, Bagent C, Catapano AL et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias; lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;111-188.
- Bangalore S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713-9.
MAT-IE-2300173 (v3.0)
Date of Preparation: April 2024




-(1).webp/jcr:content/cardiovascular%20(3)%20(1).webp)


.webp/jcr:content/cardiovascular%20(3).webp)

