Profile
Clexane® is an anticoagulant of the low-molecular-weight heparin (LMWH) class, derived from natural porcine heparin and with a wide spectrum of licenced indications.
Enoxaparin is marketed in the Republic of Ireland under the trade name Clexane.®
As a LMWH, Clexane® comprises a fragment of the naturally occurring mucopolysaccharide, heparin. It has a variable size, with an average molecular weight of 4–5 kilodaltons (kD).1
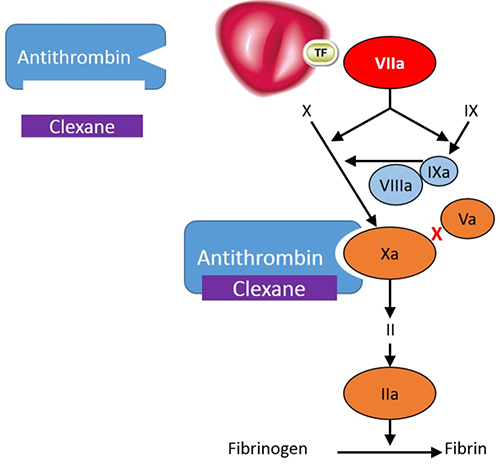
Clexane® exerts its anticoagulant effect by preventing the formation of blood clots through binding to antithrombin, a naturally occurring coagulation inhibitor and potentiating its action. Antithrombin is a natural inhibitor of the coagulation factors FXIa, FIXa, FXa and FIIa (thrombin).1
The pharmacodynamic profile of enoxaparin, like other LMWHs, has advantages over unfractionated heparin (UFH). These include:
- Minimal plasma binding, leading to more reliable anticoagulant effects and eliminating the need for therapeutic monitoring
- A greater capacity to release tissue factor pathway inhibitor and hence the generation of FVIIa
- A lower propensity to inhibit platelet aggregation
- Less inhibition by platelet factor 4
- Potential antiplatelet effects via higher degrees of suppression of von Willebrand factor
- Less propensity to cause heparin-induced thrombocytopenia and osteoporosis
Clexane® exerts its anticoagulant effect by preventing the formation of blood clots through binding to antithrombin, a naturally occurring coagulation inhibitor and potentiating its action.1
Antithrombin is a natural inhibitor of the coagulation factors FXIa, FIXa, FXa and FIIa (thrombin).
Clexane® forms a complex with antithrombin. This complex undergoes a conformational change; in its altered conformation, the complex inhibits FXa, which is the primary mechanism of action. It also inhibits FIIa, although to a lesser extent - in vitro, Clexane has a high anti-Xa activity and low anti-IIa or anti thrombin activity, with a ratio of 3.6 :1.1
In the Republic of Ireland, Clexane® has a wide range of therapeutic indications.3 Please refer to medicines.ie for more details.
Clexane® is indicated in adults for:
- Prophylaxis of venous thromboembolic disease in moderate and high risk surgical patients, in particular those undergoing orthopaedic or general surgery including cancer surgery.
- Prophylaxis of venous thromboembolic disease in medical patients with an acute illness (such as acute heart failure, respiratory insufficiency, severe infections or rheumatic diseases) and reduced mobility at increased risk of venous thromboembolism.
- Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), excluding PE likely to require thrombolytic therapy or surgery.
- Prevention of thrombus formation in extra corporeal circulation during haemodialysis.
- Acute coronary syndrome:
- Treatment of unstable angina and Non ST-segment elevation myocardial infarction (NSTEMI), in combination with oral acetylsalicylic acid.
- Treatment of acute ST-segment elevation myocardial infarction (STEMI) including patients to be managed medically or with subsequent percutaneous coronary intervention (PCI)
- Extended treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrence in patients with active cancer.
Enoxaparin sodium is contraindicated in patients with:
- Hypersensitivity to enoxaparin sodium, heparin or its derivatives, including other low molecular weight heparins (LMWH) or to any of the excipients listed in section 6.1 of the SmPC;
- History of immune mediated heparin-induced thrombocytopenia (HIT) within the past 100 days or in the presence of circulating antibodies;
- Active clinically significant bleeding and conditions with a high risk of haemorrhage, including recent haemorrhagic stroke, gastrointestinal ulcer, presence of malignant neoplasm at high risk of bleeding, recent brain, spinal or ophthalmic surgery, known or suspected oesophageal varices, arteriovenous malformations, vascular aneurysms or major intraspinal or intracerebral vascular abnormalities;
- Spinal or epidural anaesthesia or loco-regional anaesthesia when enoxaparin sodium is used for treatment in the previous 24 hours.
Since LMWHs are eliminated predominantly via the kidneys, drug accumulation risk exists in patients with renal function impairment. Clexane® has been investigated in studies in patients with renal function impairment of various severity grades, and the Summary of Product Characteristics offers clear recommendations for dosing.2
In the steady-state, linear correlation exists between the anti-Xa plasma clearance and creatinine clearance, which indicates decreased clearance of Clexane® in patients with impaired renal function. In mild to moderate renal function impairment (creatinine clearance 50-80 ml/min or 30-50 ml/min), in the steady state after repeated subcutaneous administration of 40 mg per day the anti-Xa exposition measured as AUC is marginally increased. In those with moderate and mild renal impairment, although no dosage adjustments are recommended in patients with moderate renal impairment (creatinine clearance 30-50 ml/min) or mild renal impairment (creatinine clearance 50-80 ml/min), careful clinical monitoring is advised.
In patients with severe renal function impairment (creatinine clearance 15-30ml/min), in the steady state after repeated subcutaneous administration of 40 mg per day, the AUC is significantly increased by an average of 65%. Therefore, in patients with severe renal failure, the dose of the dose of Clexane® needs to be adjusted, as per the table below. Enoxaparin is not recommended for patients with end stage renal disease (CrCI <15ml/min).2
| Indication | Dosing regimen |
| Prophylaxis of venous thromboembolic disease | 2,000 IU (20 mg) SC once daily |
| Treatment of DVT and PE |
100 IU/kg (1 mg/kg) body weight SC once daily |
| Treatment of unstable angina and NSTEMI | 100 IU/kg (1 mg/kg) body weight SC once daily |
| Treatment of acute STEMI (patients under 75) | 1 x 3,000 IU (30 mg) IV bolus plus 100 IU/kg (1 mg/kg) body weight SC and then 100 IU/kg (1 mg/kg) body weight SC every 24 hours |
| Treatment of acute STEMI (patients under 75) | No IV initial bolus, 100 IU/kg (1 mg/kg) body weight SC and then 100 IU/kg (1mg/kg) body weight SC every 24 hours |
| Extended treatment of DVT and PE in patients with active cancer: | 100 IU/kg (1 mg/kg) body weight SC once daily |
SC, subcutaneous; IV, intravenous;
The recommended dosage adjustments do not apply to the haemodialysis indication. Although no dose adjustment is recommended in patients with moderate (creatinine clearance 30-50 ml/min) and mild (creatinine clearance 50-80 ml/min) renal impairment, careful clinical monitoring is advised.
Monitoring
In general, because response to Clexane® at usual doses is consistent from patient to patient, the anticoagulant response to enoxaparin does not need to be monitored. LMWHs have a predictable dose-response relationship and Clexane® has a half-life that permits once-or twice-daily dosing. This facilitates simple fixed or weight-based dosing, enabling outpatient treatment in appropriate cases without the need for routine monitoring. This is reflected in the Summary of Product Characteristics.2
Changes in blood clotting parameters such as thrombin time or activated partial thromboplastin time (aPTT) do not display a linear correlation with antithrombotic activity. Therefore these parameters are not suitable for monitoring purposes. However, anticoagulant activity of enoxaparin can be monitored by measuring factor Xa inhibition (anti-factor Xa activity). The plasma anti-Xa assay is a laboratory test that indirectly measures the activity of heparins.3 It is predominantly used for monitoring patients treated with low molecular weight heparins, particularly when dosing at the extremes of weight and in patients who are pregnant, critically ill or have renal impairment.3
This monitoring is controversial as there is a poorly defined therapeutic range in different clinical settings and with different dosing regimens. Consequently, the timing of blood tests and their interpretation is problematic, often resulting in empirical dosing strategies.3
As there is a risk of antibody-mediated heparin-induced thrombocytopenia occurring with low molecular weight heparins, regular platelet count monitoring should be considered prior to and during therapy with these agents. Thrombocytopenia, should it occur, usually appears between the 5th and the 21st day following the initiation of therapy and may be complicated by thrombosis. Therefore, it is recommended that the platelet counts be measured before the initiation of therapy with Clexane® and then regularly thereafter during the treatment. In practice, if a confirmed significant decrease of the platelet count is observed (30 to 50% of the initial value), enoxaparin sodium treatment must be immediately discontinued and the patient switched to another therapy.3
Additional monitoring may be required for:
- patients older than 75 years treated for STEMI
- patients with renal impairment
- low weight patients
- obese patients
Please refer to the SmPC for the full list of monitoring requirements, special warnings and precautions for use.
Overdose
Accidental overdose with enoxaparin sodium after IV, extracorporeal or SC administration may lead to haemorrhagic complications. Following oral administration of even large doses, it is unlikely that enoxaparin sodium will be absorbed.
The anticoagulant effects can be largely neutralized by the slow IV injection of protamine sulfate. The dose of protamine sulfate depends on the dose of enoxaparin sodium injected; 1 mg protamine sulfate neutralizes the anticoagulant effect of 100 IU (1 mg) of enoxaparin sodium, if enoxaparin sodium was administered in the previous 8 hours.
- An infusion of 0.5 mg protamine sulfate per 100 IU (1 mg) of enoxaparin sodium may be administered if enoxaparin sodium was administered greater than 8 hours previous to the protamine sulfate administration, or if it has been determined that a second dose of protamine sulfate is required.
- After 12 hours of the enoxaparin sodium injection, protamine sulfate administration may not be required. However, even with high doses of protamine sulfate, the anti-Xa activity of enoxaparin sodium is never completely neutralized (maximum about 60%) (see the prescribing information for protamine sulfate salts).
Please see pages 13 and 14 of the SmPC for more information.
Administration
There are several different doses of Clexane®, each dose has a different label colour.
To identify the corresponding syringe. When using Clexane,® colour alone should not be used to distinguish syringes – the patient should be advised to always read labels carefully. Further information is available in the Summary of Product Characteristics.3

References:
- Carter NJ, McCormack PL, Plosker GL. Enoxaparin: a review of its use in ST-segment elevation myocardial infarction. Drugs 2008; 68:691–710.
- Clexane Summary of Product Characteristics
- Barras M. Anti-Xa assays. Aust Prescr 2013; 36:98-101.
MAT-IE-2500207 (v1.0)
Date of Preparation: January 2026