- Article
- Source: Campus Sanofi
What is the efficacy and safety profile of ezetimibe/rosuvastatin combination?
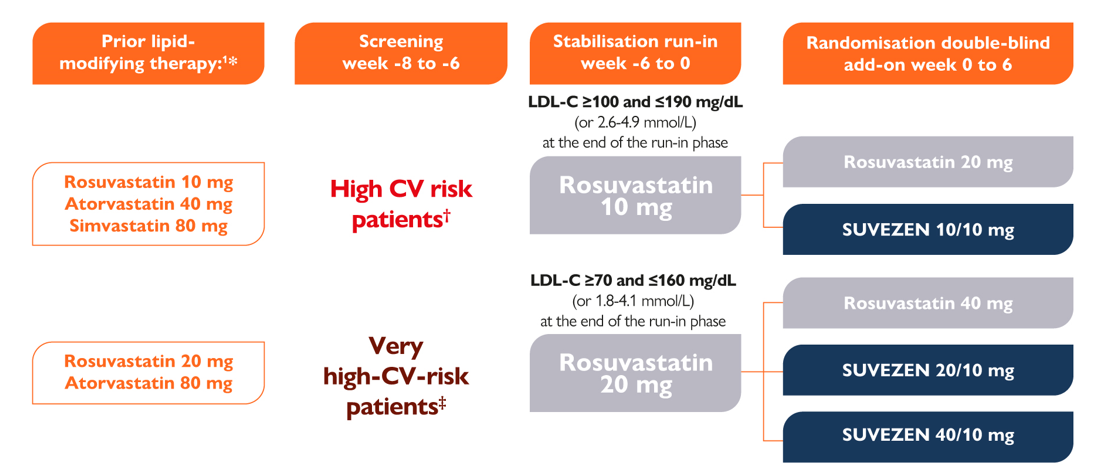
Add-on Trial Study Design1
All patients were on prior stable dose of statin and, following this, high CV risk patients were stabilised for 6 weeks with rosuvastatin 10 mg and very-high CV risk patients with rosuvastatin 20 mg1
After stabilisation with rosuvastatin, patients received either Suvezen (rosuvastatin + ezetimibe) or rosuvastatin up-titration:
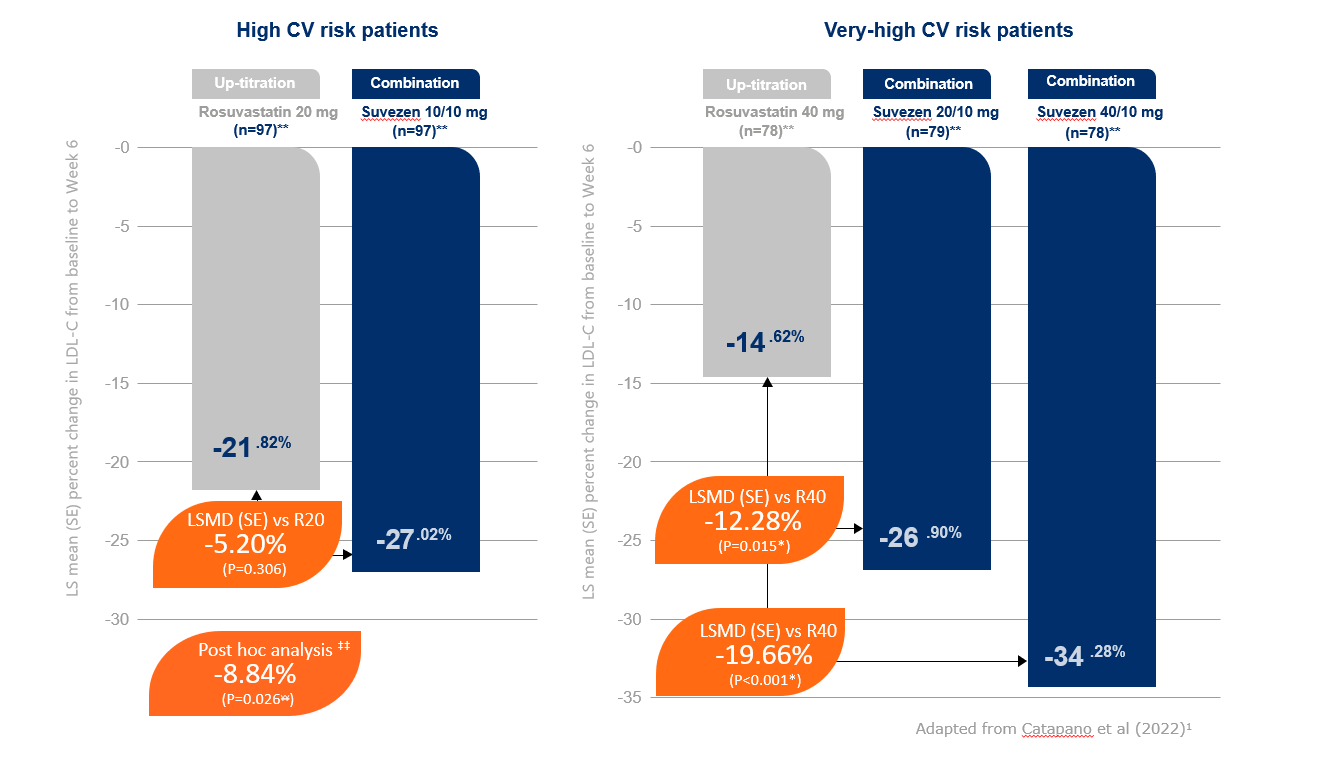
Suvezen demonstrated a greater reduction in LDL-C compared to rosuvastatin up-titration (from baseline to week 6 in patients inadequately controlled with statin alone and at very high risk)
A numerically greater reduction in LDL-C compared to rosuvastatin up-titration was observed (from baseline to week 6 in patients inadequately controlled with statin alone and at high risk)1,2
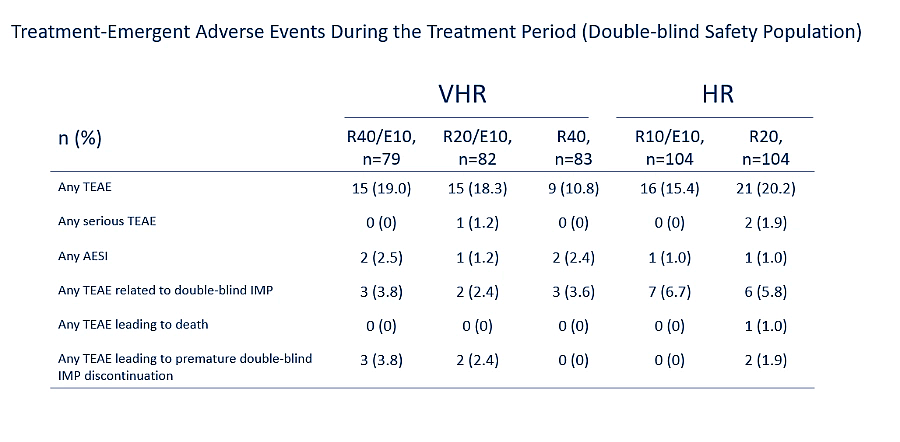
Overall safety findings observed in patients treated with all three strengths of Suvezen in this study were consistent with known safety profile of rosuvastatin and ezetimibe1,2
Add-on Trial1: A multi-centre, multinational, randomized, double-blind, double-dummy, active-controlled, parallel-arm study of FDC R/E in people with primary hypercholesterolemia at very high risk (VHR) or high risk (HR) of CVD, inadequately controlled with 20 mg or 10 mg stable daily dose of rosuvastatin or equipotent dose of another statin. The primary objective was to demonstrate superiority of FDC R/E versus rosuvastatin monotherapy uptitrated to 40 mg (R40) or 20 mg (R20) in reduction of LDL-C after 6 weeks.
* Prior defined as used from 6 weeks before screening visit to first dose of study treatment1
** lnitial numbers of patient population randomised were as follows: R20 (n=104), Suvezen (FDC R10/E10) (n=104), R40 (n=83), Suvezen (FDC R20/E10) (n=82) and Suvezen (FDC R40/E10) (n=79)
¶ mITT population
† High CV risk defined as those with any of the following: markedly elevated single risk factor, in particular cholesterol >8 mmol/L (310 mg/dL) or blood pressure ≥180/110 mmHg; diabetes mellitus patients other than those at very high-CV-risk (with the exception of young patients with type 1 diabetes mellitus and patients without major risk factors being at low or moderate CV risk); moderate CKD (GFR ≥30–≤59 mL/min/1.73m2); a SCORE value of ≥5%–<10% for 10-year risk of first fatal CVD1
‡ Very high-CV-risk defined as those with any of the following: documented clinical CVD; unequivocally documented CVD on imaging including significant plaque on coronary angiography or carotid ultrasound; diabetes mellitus with target organ damage such as proteinuria or with a major risk factor such as smoking. marked hypercholesterolaemia, or marked hypertension; severe chronic kidney disease (CKD; GFR <30 mL/min/1.73m2); a SCORE value for 10-year risk of first fatal CVD of ≥10% prior to commencing LMT1
¥¥ Statistically significant per fixed hierarchical approach used to ensure strong control of overall type-I error rate at 0.05
‡‡ After exclusion of data from one outlier participant in the FDC R10/E10 arm
- Catapano A, et al. A Phase 3 Randomized Controlled Trial to Evaluate Efficacy and Safety of New-Formulation Zenon (Rosuvastatin/Ezetimibe Fixed-Dose Combination) in Primary Hypercholesterolemia Inadequately Controlled by Statins. J Cardiovasc Pharmacol Ther. 2022; 27: 1-11.
- Suvezen (Rosuvastatin and ezetimibe) Summary of Product Characteristics. Available at https://www.medicines.ie/medicines/list/all/page-1/per-page-25?query=suvezen.
AESI, adverse event of special interest; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; E, ezetimibe; E10, ezetimibe 10 mg; FDC, fixed dose combination; HR, high risk; IMP, investigational medicinal product; LDL-C, low-density lipoprotein cholesterol; LS, least squares; LSMD, least squares mean difference; mg, milligram; mITT, modified intention-to-treat; n, number of participants; R, rosuvastatin; R40, rosuvastatin 40 mg; SCORE, systematic coronary risk estimation; TEAE, treatment-emergent adverse event; VHR, very high risk.
Our Products