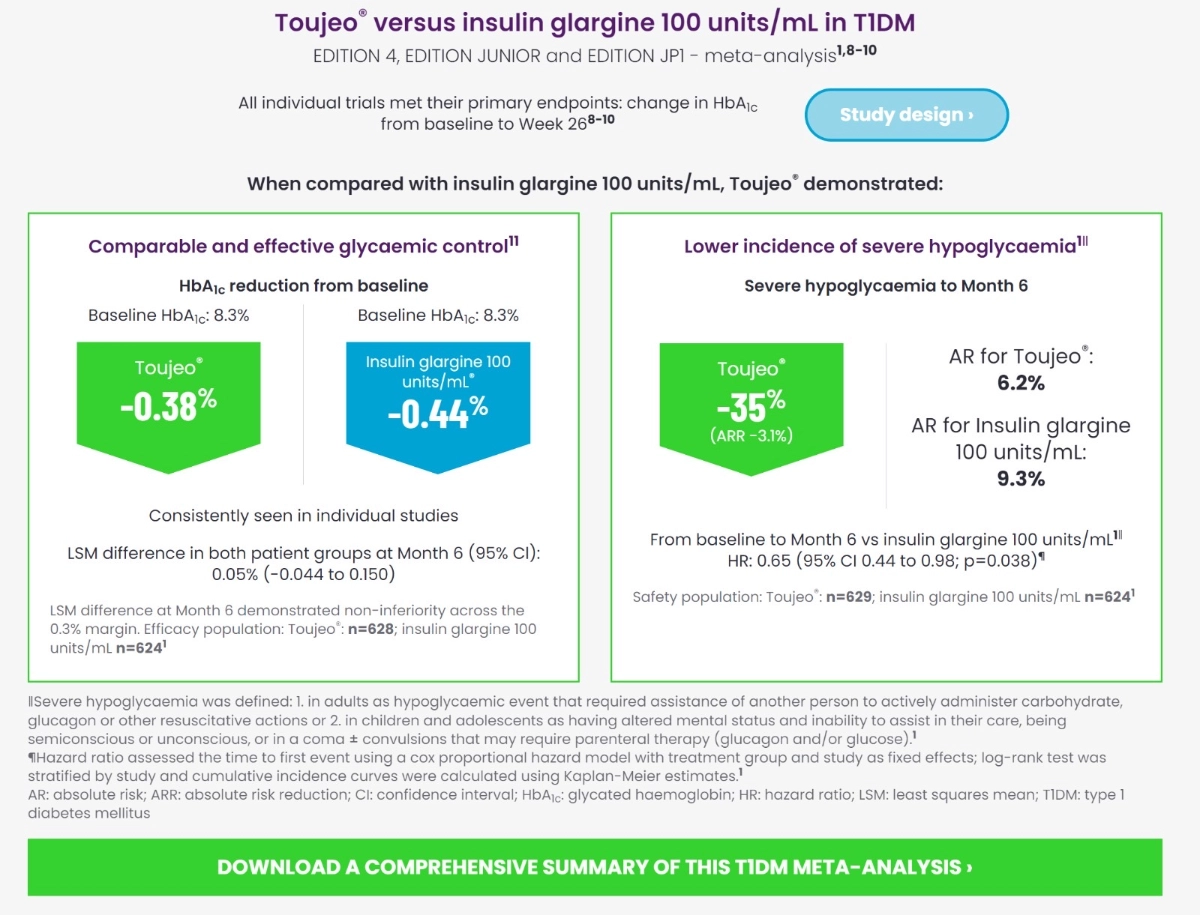
Toujeo® (insulin glargine 300 Units/mL) Versus insulin glargine 100 units/mL in T1DM

Clinical summary to read about the benefits of Toujeo® in children and adolescents from the age of 6 years in this randomised trial
Hypoglycaemia is the most frequent adverse reaction observed in clinical trials conducted with Toujeo®.2 Other common adverse effects are lipohypertrophy and injection site reactions.2
For further information on the safety profile of Toujeo® please consult the Summary of Product Characteristics >
- Danne T, Matsuhisa M et al. Lower risk of severe hypoglycaemia with insulin glargine 300 U/mL versus glargine 100 U/mL in participants with type 1 diabetes: a meta-analysis of 6-month phase 3 clinical trials. Diabetes Obes Metab 2020;22(10):1880-1885
- Toujeo® Summary of Product Characteristics. Available at: www.medicines.ie (Accessed July 2024)
- Danne T, Tamborlane W V et al. Efficacy and safety of insulin glargine 300 units/ml (gla-300) versus insulin glargine 100 units/ml (gla-100) in children and adolescents (6-17 years) with type 1 diabetes: results of the EDITION JUNIOR randomised controlled trial. Diabetes Care 2020;43(7):1512-1519
- Home P D, Bergenstal R M et al. New insulin glargine 300 units/ml versus glargine 100 units/ml in people with type 1 diabetes: a randomised, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care 2015;38(12):2217-2225
- Matsuhisa M, Koyama M et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomised controlled trial (EDITION JP 1). Diabetes Obes Metab 2016;18(4):375-383
- Danne T, Matsuhisa M et al. Lower risk of severe hypoglycaemia with insulin glargine 300 U/mL versus glargine 100 U/mL in participants with type 1 diabetes: a meta-analysis of 6-month phase 3 clinical trials. Diabetes Obes Metab 2020;22(10):1880-1885 (suppl)
ARR: Absolute Risk Reduction
HR: Hazard Ratio
OR: Odds Ratio
RR: Relative Risk
T1D – Type-1 Diabetes
T2D – Type -2 Diabetes
Diabetes Products


MAT-IE-2300505 (v1.0)
Date of Preparation: July 2024

.webp/jcr:content.webp)