Benefits of switching to Toujeo® (insulin glargine 300 Units/mL) through patient case studies and patient reported outcomes
T1 Case Studies
T2 Case Studies
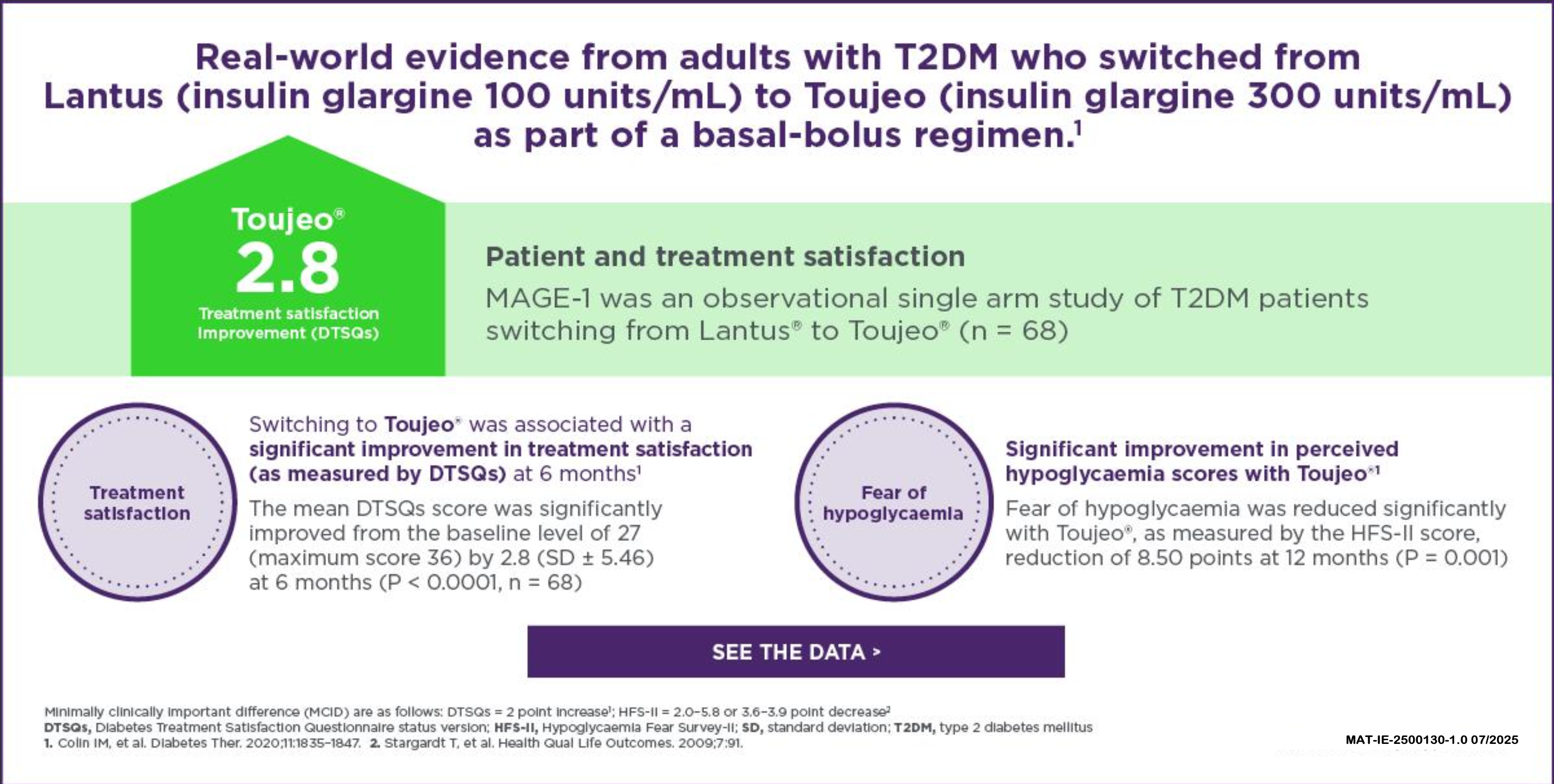
Real-World Evidence
Hypoglycaemia is the most frequent adverse reaction observed in clinical trials conducted with Toujeo®.7 Other common adverse effects are lipohypertrophy and injection site reactions.7
For further information on the safety profile of Toujeo® please consult the Summary of Product Characteristics >
1 Colin IM, et al. Diabetes Ther. 2020;11:1835–1847
7 Toujeo® Summary of Product Characteristics. Available at: www.medicines.ie (Accessed September 2025)
Diabetes Products


MAT-IE-2300506 (v2.0)
Date of Preparation: September 2025

.webp/jcr:content.webp)
.svg/jcr:content.png)
.svg/jcr:content.png)
.svg/jcr:content.png)
.svg/jcr:content.png)
.svg/jcr:content.png)
.svg/jcr:content.png)
.svg/jcr:content.png)
.svg/jcr:content.png)
.svg/jcr:content.png)
.svg/jcr:content.png)
.svg/jcr:content.png)