Disease Overview
Smoldering disease is a key unaddressed component of MS, driving disease progressions1-3
The Silent Progression in MS: Smoldering neuroinflammation and PIRA
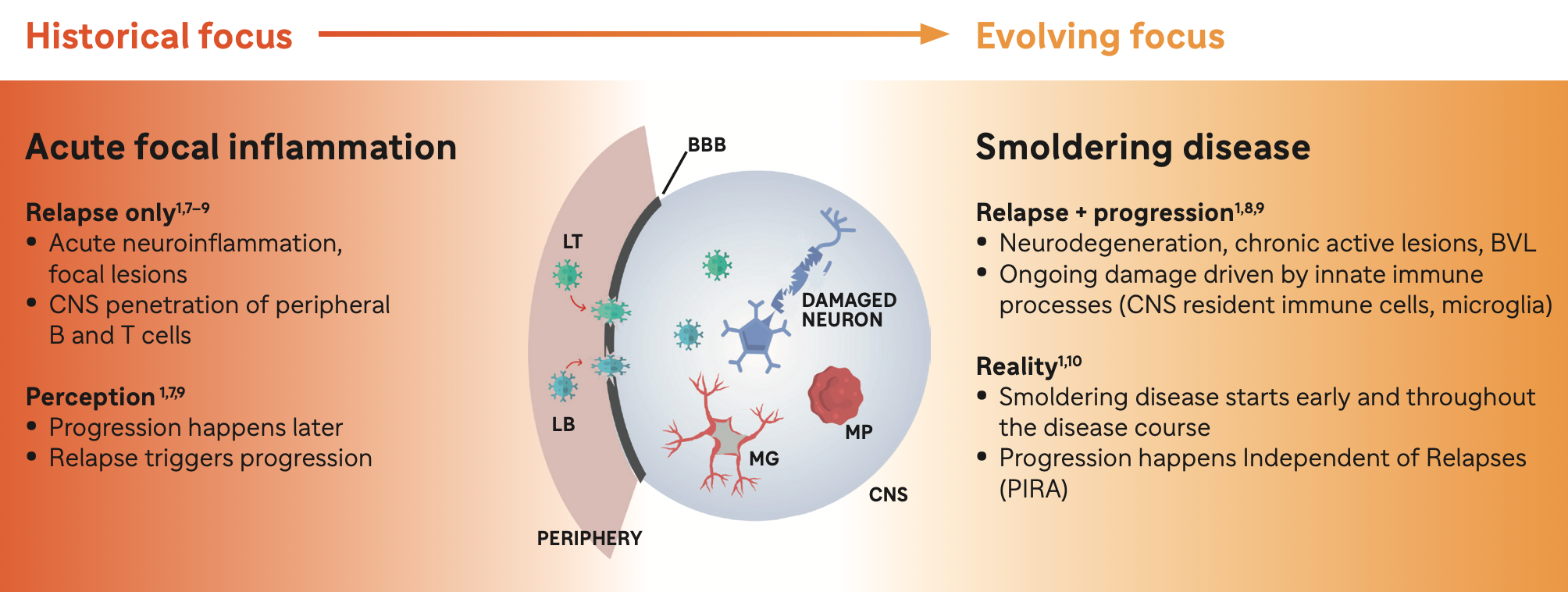
Acute Neuroinflammation
Acute neuroinflammation is driven in party by activated B cells and T cells derived from the periphery
• Leads to relapses,acute lesions, and RAW1,8,11

10%-20% of disability accumulation in treated patients is driven by relapses and acute lesions 12,13
Smoldering Neuroinflammation

Smoldering neuroinflammation is driven in primarily by disease-associated microglia found in the CNS1,8,11
• Leads to PIRA,resulting in physical disability and cognitive worsening1,8,11
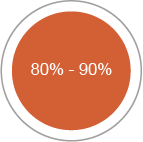
80%-90% of disability accumulation in treated patients is relapse-independent and driven by smoldering neuroinflammation.12,13
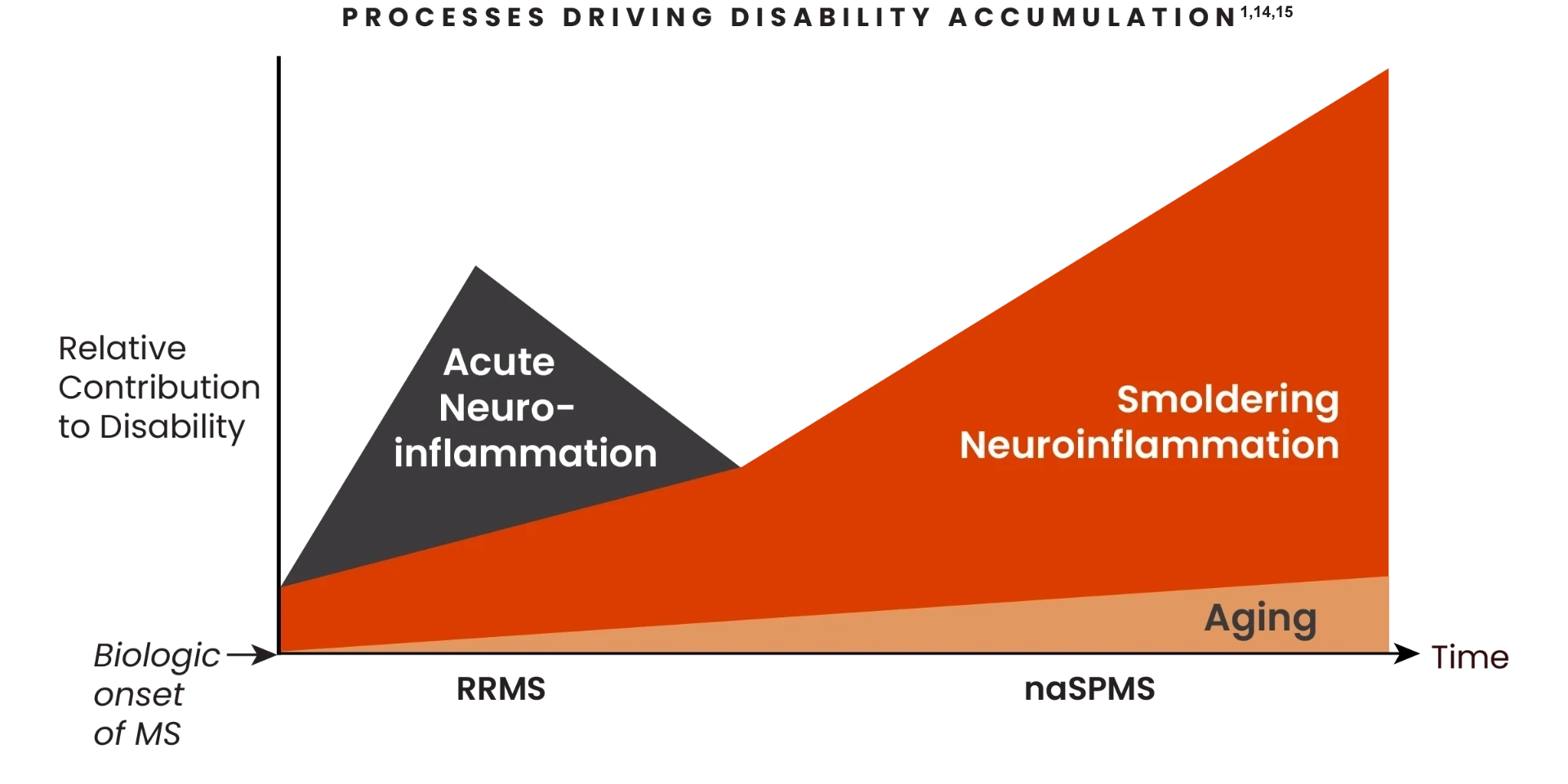
Both concurrent neuroinflammatory processes drive disability accumulation in MS 1,14-16
Smoldering neuroinflammation starts at disease onset and increasingly drives disability accumulation1
The relative contribution of both acute and smoldering neuroinflammation shifts as patients progress from RRMS to nrSPMS1,14
- Smoldering neuroinflammation presents early and throughout:the spectrum of MS, before initial clinical symptoms.1,11
- Over the course of MS, acute neuroinflammation may decline, but smoldering neuroinflammation persists in the absence of relapses and acute lesions1
- Disability accumulation driven by smoldering neuroinflammation can:
- Happen throughout the disease spectrum9
- Affect any patient, regardless of the type of MS or if patients have a history of taking DMTs1
- Smoldering neuronflammation is driven primarily by disease-associated microglia found in the CNS and contributes to both physical and cognitive disability accumulation1,17
For more information about Acute and Smoldering Inflammation, click on this link:
BBB, blood-brain barrier; BVL, brain volume loss; CNS, central nervous system; LB, B lymphocyte; LT, T lymphocyte; MG, microglia; MP; macrophage; MS, multiple sclerosis.
- Giovannoni G et al. Ther Adv Neurol Disord 2022; 15: 1–18.
- Balasa R et al. Int J Mol Sci 2021; 22(16): 8370.
- Bierhansl L et al. Nat Rev Drug Discov 2022; 21(8): 578–600.
- Lassmann H. Front Immunol 2019; 9: 3116.
- Rissanen E et al. Neurol Neuroimmunol Neuroinflamm 2018; 5: e443.
- Elliott C et al. Brain 2019; 142(9): 2787–2799.
- Lublin FD et al. Neurol 2014; 83 :278–286.
- Häusser-Kinzel S, Weber MS. The role of B cells and antibodies in multiple sclerosis, neuromyelitis optica, and related disorders. Front Immunol. 2019;10:201. doi:10.3389/fimmu.2019.00201
- Cree BAC et al. Ann Neurol 2019; 85(5): 653–666.
- Kappos L et al. Mult Scler 2018; 24: 963–973
- Giovannoni G. The neurodegenerative prodrome in multiple sclerosis. Lancet Neurol. 2017;16(6):413-414.
- Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132-1140.
- Ingwersen J, Masanneck L, Pawlitzki M, et al. Real-world evidence of ocrelizumab-treated relapsing multiple sclerosis cohort shows changes in progression independent of relapse activity mirroring phase 3 trials. Sci Rep. 2023;13(1):15003. doi:10.1038/s41598-023-40940-w
- Filippi M, Amato MP, Centonze D, et al. Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: an expert opinion. J Neurol. 2022;269(10):5382-5394.
- Cree BAC, Hollenbach JA, Bove R, et al; University of California, San Francisco MS-Epic Team. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653-666.
- Kreiger SC, Antoine A et al. EDSS 0 is not normal: multiple sclerosis disease burden below the clinical threshold. Mult Scler. 2022;28(14):2299-2303
- Correale J, Halfon MJ, Jack D, Rubstein A, Villa A. Acting centrally or peripherally: a renewed interest in the central nervous system penetration of disease-modifying drugs in multiple sclerosis. Mult Scler Relat Disord. 2021;56:103264.doi 10.1016/j.msard.2021.103264