오바지오® 요양급여
- Patric Vermersch at el., Multiple Sclerosis and Related Disorders Vol 43. 2020. 102158.
- 보건복지부 고시 제2015-154호.
- 보건복지부 고시 제2021-53호.
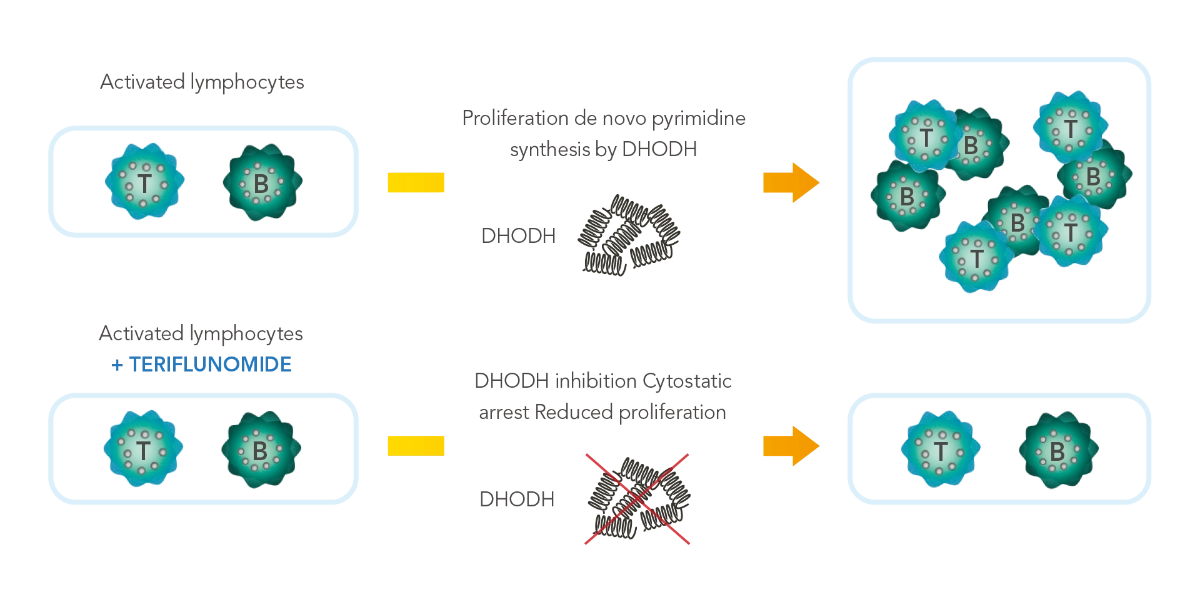
오바지오®의 작용기전
오바지오®는 MS 병인과 관련된 염증반응을 일으키는 활성화된 T 림프구와 B 림프구의 증식을 억제하는 면역조절제입니다.1,#
- 오바지오®는 활성화된 림프구에서 높은 수준으로 발현하는 미토콘드리아 효소인 DHODH에 특이적으로 비경합적/가역적 억제를 하여 DNA 합성에 필요한 de-novo pyrimidine 합성을 차단합니다.1,#
- 오바지오®는 비활성화 된 림프구에는 영향을 미치지 않습니다.1,#
- 오바지오®는 림프구 증식을 선별적, 가역적으로 줄여 정상적인 면역 반응을 유지하도록 도와줍니다.1,#
MS, Multiple sclerosis; DHODH , dihydro-orotate dehydrogenase; DNA, Deoxyribonucleic acid; MoA, Mechanism of action
- Bar-Or A, et al. Drugs. 2014;74(6):659-674.
# 오바지오의 작용기전은 명확히 밝혀지지 않았습니다.
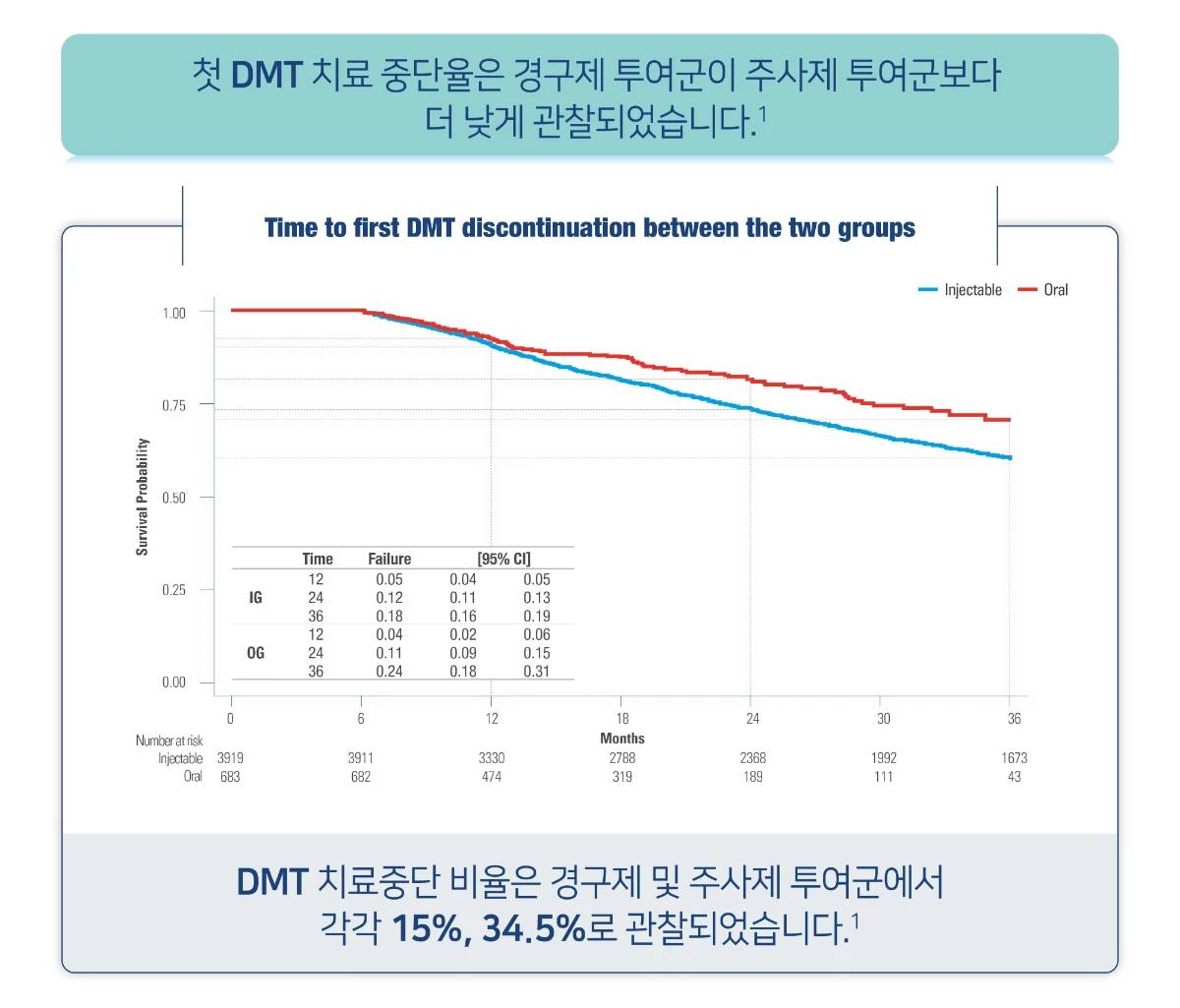
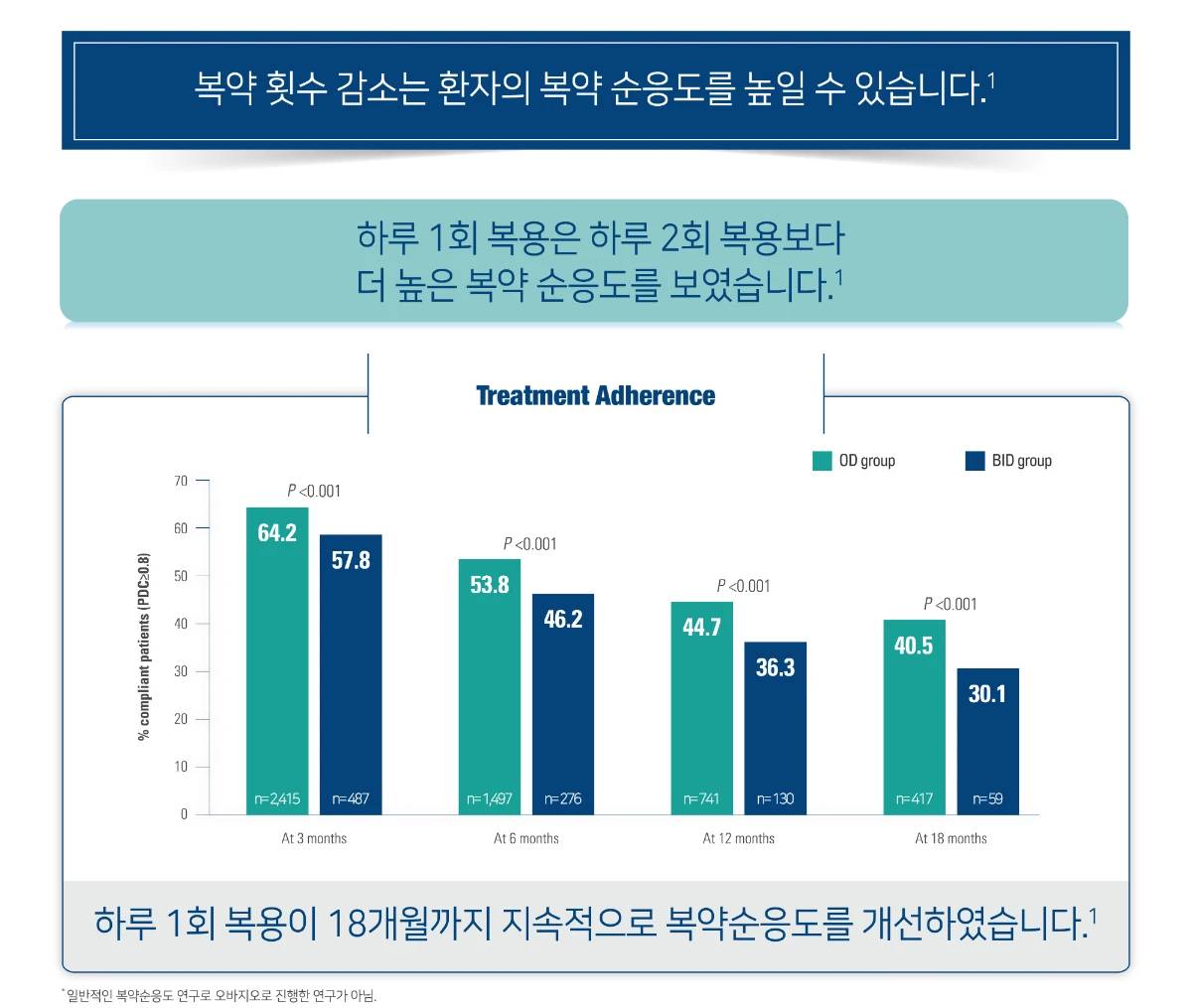
오바지오® 복약순응도
CI, confidence interval; DMT, disease-modifying therapy; IG, injectable group; OG, oral group; RRMS, relapsing-remitting multiple sclerosis.
[Study Design]
This multicenter, observational, retrospectively acquired, and propensity adjusted cohort study utilized RRMS-naïve patients from the Italian MS Register who started either injectable or oral first-line DMTs between January 1, 2010, and December 31, 2017, to evaluate the impact on disability outcomes in patients. Enrolled patients were divided into two groups, namely the injectable group (IG) and the oral group (OG). Of a cohort of 11,416 patients, 4602 were enrolled (3919 in the IG and 683 in the OG). RRMS-naïve patients who matched the required criteria were divided into two groups for the analyses, the injectable group (IG) and oral group (OG). The IG included RRMS patients who were treated with either Copaxone (40 mg per ml/three times per week subcutaneously and at least 48 h apart) or IFNs (interferon β-1a and interferon β-1b, 30 μg/0.5 mL, once weekly, intramuscularly or interferonβ-1a, either 22 mcg or 44 mcg, three times per week subcutaneously). The OG included RRMS patients who were treated with either DMF (120 mg twice per day for the first 7 days, then 240 mg twice per day) or TRF (14 mg once per day). The IG had a higher rate of women (67.3%vs 63.4%, < 0.05) and a lower mean age (36.1 ± 10.9 vs 38.9 ± 11.8, < 0.001).
- Emanuele D’Amico, et al. Neurotherapeutics, 2021;18 905-919.
- Patric Vermersch, et al. Mult Scler Relat Disord, 2020 Aug;43:102158.
BID, twice daily; CI, confidence interval; DMT, disease-modifying therapy;OD, once-daily; PDC, proportion of days covered; RRMS, relapsing-remitting multiple sclerosis.
[Study Design]
It analyzed the PharMetrics Integrated Claims database (claims of commercial insurers in the US) from 1 January 2004, through 31 December 2009. Adult patients with continuous insurance coverage, newly initiated on diabetes mellitus or hypertension medication, and having at least one VTE diagnosis were included. Adherence to OD and BID therapies was calculated by using two measures: medication possession ratio (MPR) and proportion of days covered (PDC). Adherence was defined as an MPR or PDC≥0.8. Multivariate logistic regressions were conducted to compare the probability of adherence between the OD and BID groups adjusting for baseline confounders.
- Laliberte F, et al. Patient. 2013;6 213-224.
- Patric Vermersch, et al. Mult Scler Relat Disord, 2020 Aug;43:102158.
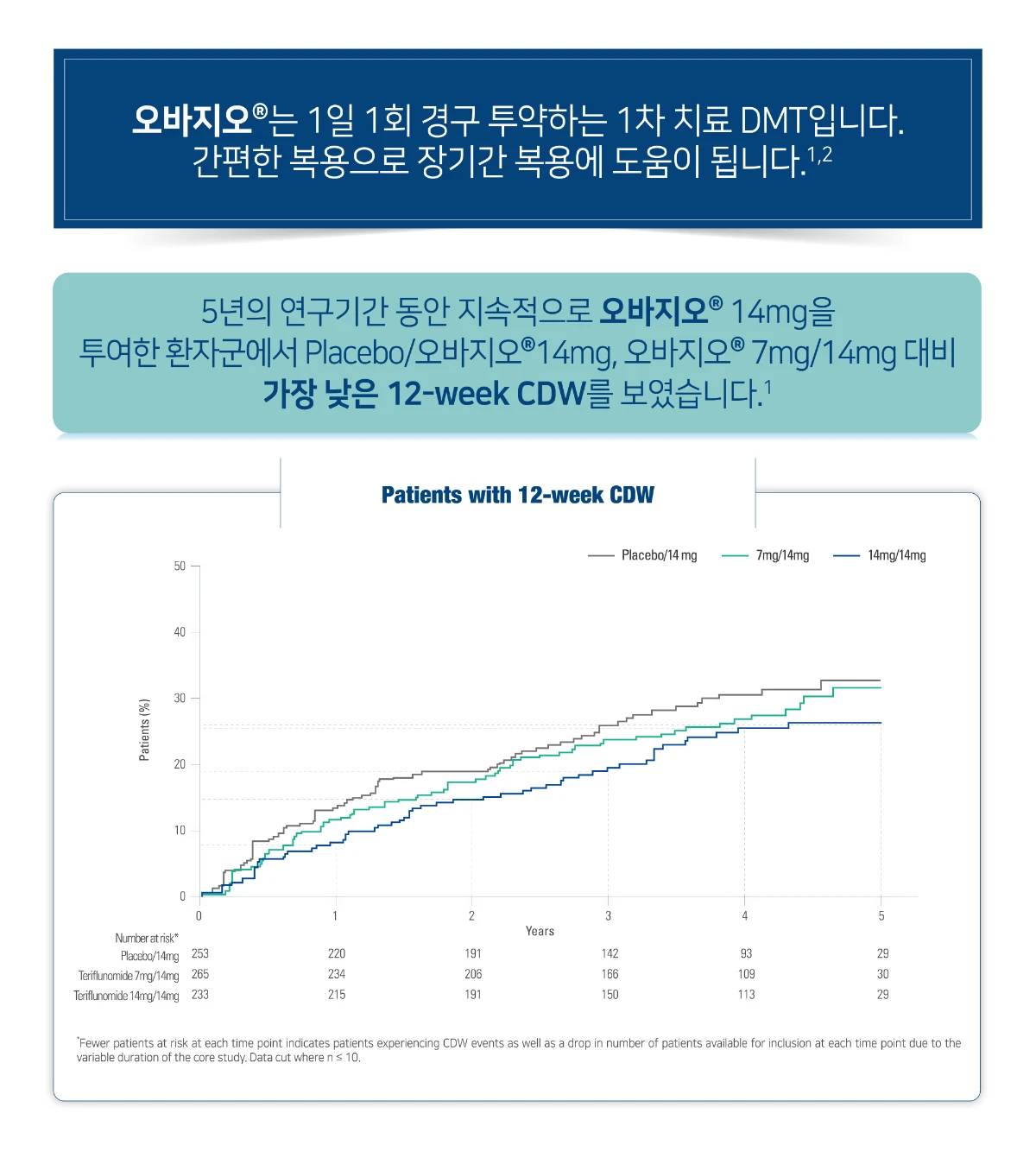
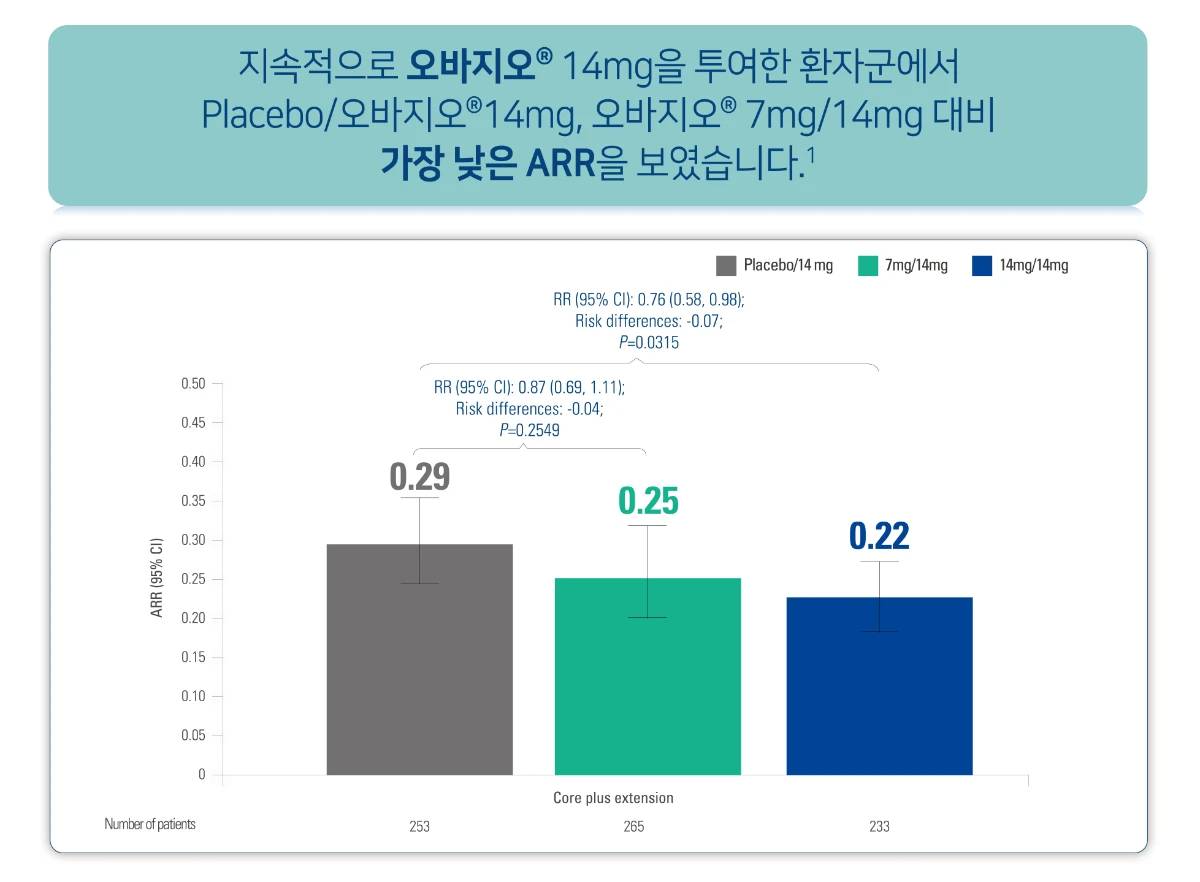
오바지오®의 효능
ARR, annualized relapse rate; CDW, confirmed disability worsening; CI, confidence interval; DMT, disease-modifying therapy; RR, relative risk; RMS, relapsing forms of multiple sclerosis.
[Study Design]
A randomized, double-blind, placebo-controlled, phase 3 study in patients with RMS. Patients were randomly assigned 1:1:1 to receive placebo, teriflunomide 7 mg, or teriflunomide 14 mg until a fixed time point 48 weeks after the last patient was randomly assigned. Patients received treatment for a maximum of 152 weeks, depending on time of enrollment, before core study end. Patients who completed the core study were invited to enter the open-label long-term extension. Patients who received teriflunomide 14 mg in the core study continued on their original dose (14 mg/14 mg group); patients who received placebo or teriflunomide 7 mg in the core study were reassigned to teriflunomide 14 mg (placebo/14 mg and 7 mg/14 mg groups, respectively). The duration of treatment in the extension was variable; patients were considered to have completed the extension when commercial teriflunomide became available in their region or when the study stopped. The pharmacist or treating physician determined treatment compliance by examining the administered blister packs for used and unused doses.
The Bigger Picture – an overview of Aubagio
본 영상은 오바지오®의 효과와 안전성 프로파일을 소개하며, 뇌 부피 감소와 장애 누적에 대한 정보 또한 포함하고 있습니다.
The Bigger Picture – an overview of Aubagio
Disability Progression/Worsening Short Video
본 영상은 오바지오®가 장애 진 및 악화를 지연시키는 효능에 대한 설명을 제공합니다.
Disability Progression/Worsening Short Video
2023 AOK AUBAGIO Positioning teriflunomide in Current Therapeutic Landscape
본 영상은 하단의 세 가지 주제를 통해 오바지오®에 대한 정보를 전달합니다.
1. Aubagio in treatment-naive patients
2. Treatment switch
3. Aubagio across the age
2023 AOK AUBAGIO Positioning teriflunomide in Current Therapeutic Landscape
- Aron E Miller, et al. Mult Scler Relat Disord. 2020;46:102438.
- Patric Vermersch, at el. Mult Scler Relat Disord. Vol 43. 2020. 102158.
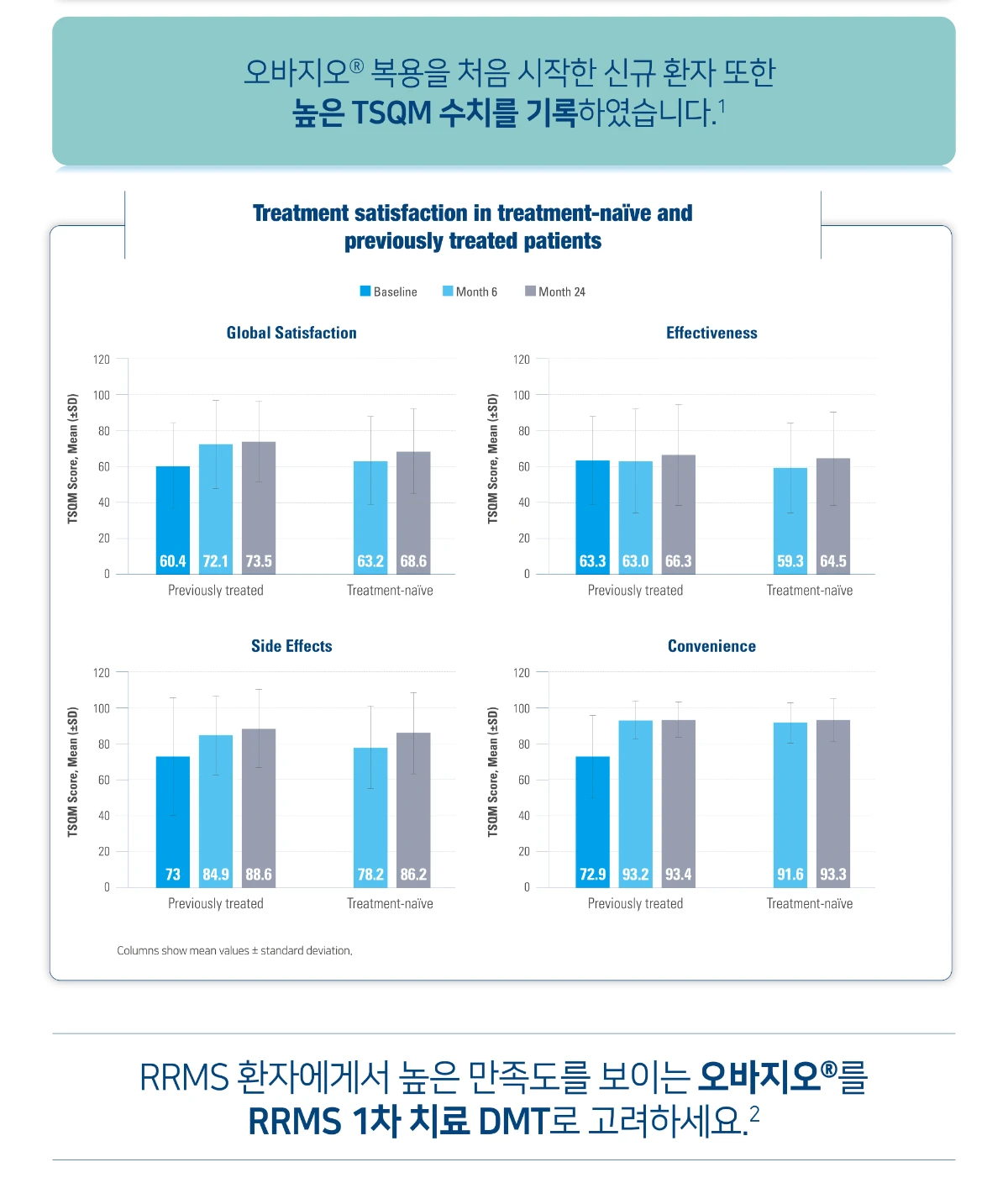
오바지오®의 환자 만족도
DMT, disease-modifying therapy; RRMS, relapsing-remitting multiple sclerosis; SD, standard deviation; TSQM, Treatment Satisfaction Questionnaire for Medication.
[Study Design]
Teri-LIFE was a prospective, open label, non-interventional, observational, multi-centre study that enrolled 200 teriflunomide-treated patients from three Nordic countries(Denmark, Norway and Sweden). The primary outcome measure changes in patient-reported QoL over 24 months as measured by the Short Form-36 (SF-36) questionnaire. Secondary endpoints included clinical efficacy, fatigue, safety, treatment satisfaction (Treatment Satisfaction Questionnaire for Medication version 1.4 (TSQM-1.4)), treatment adherence, and health economic outcomes. Most assessments were made at baseline and then at 6-monthly intervals.
- ALK Hestvik, et al. Multiple Sclerosis and Related Disorders. 2022;103892.
- Vermersch P, et al. Multiple Sclerosis and Related Disorders. 2020;43:102158.
제품을 처방하기 전에 반드시 국내 허가사항을 확인하시기 바랍니다.
MAT-KR-2202147-1.0/11/2022





.webp)


.jpg/jcr:content/image%20(58).jpg)