비용종을 동반한 만성 비부비동염(CRSwNP)의 일상생활에서의 증상 부담












[Study design2] This study was the impact of dupilumab on sense of smell in severe CRSwNP. In the randomized SINUS-24 and SINUS-52 studies, adults with severe CRSwNP received dupilumab 300 mg subcutaneously or matching placebo every 2 weeks for 24 or 52 weeks, respectively. Smell was assessed using daily patient-reported LoS score (0-3) and University of Pennsylvania Smell Identification Test (UPSIT; 0-40). Data from the 2 studies were pooled through week 24. Relationships between patient phenotypes and smell outcomes were also assessed.
[Study design3] This study was identify associations between inflammatory endotypes and clinical presentations in CRS. Compared 121 patients with nonpolypoid CRS (CRSsNP) and 134 patients with polypoid CRS (CRSwNP) and identified inflammatory endotypes using markers including IFN-γ (T1), eosinophil cationic protein (T2), Charcot-Leyden crystal galectin (T2), and IL-17A (T3). We collected clinical parameters from medical and surgical records and examined whether there were any associations between endotype and clinical features.
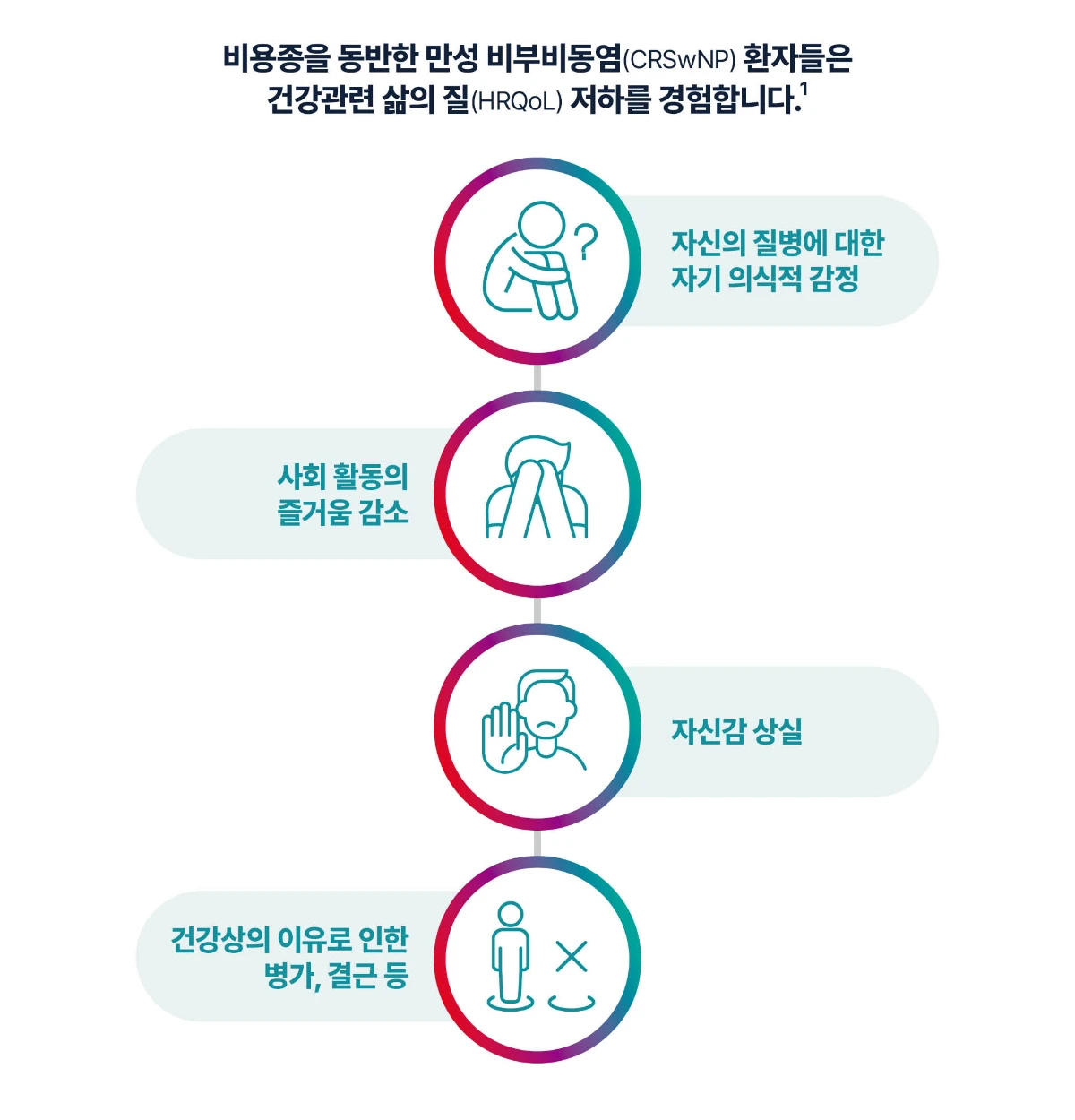
[Study design4] This study was identify associations between Impact of CRSwNP on QoL. Investigators conducted a systematic primary and secondary Medline and PubMed search for data on QoL and CRSwNP published between January 2010 and March 2021. Five separate searches captured key QoL data.
[Study design5] This study sought to determine whether individual symptom clusters differentially impact the likelihood of depression in a cohort of CRSwNP patients. Retrospectively included 77 patients with CRSwNP. The severity of sinonasal symptoms was assessed using the 22-item Sino-Nasal Outcome Test (SNOT-22) and grouped according to a previously validated four-subdomain structure: nasal, otologic/facial pain, sleep, and emotional subdomains. The likelihood of major depressive disorders was assessed using the Patient Health Questionnaire-2 (PHQ-2). The clinical characteristic of symptom severity (nasal polyp size) and disease-specific information, such as the number of previous sinonasal surgeries, were also collected.
[Study design7] Investigators conducted a systematic literature review (SLR) to determine the epidemiology and clinical burden of chronic rhinosinusitis with nasal polyps (CRSwNP) and to describe how the addition of biologics has affected outcomes for patients with CRSwNP. Relevant studies published between 1 January 2008 and 8 February 2019, for epidemiology, and 1 January 2008 and 16 February 2019, for clinical burden, and relevant conference abstracts from 1 January 2017 to 7 March 2019, for epidemiology and 1 January 2017-16 February 2019 for clinical burden were included.
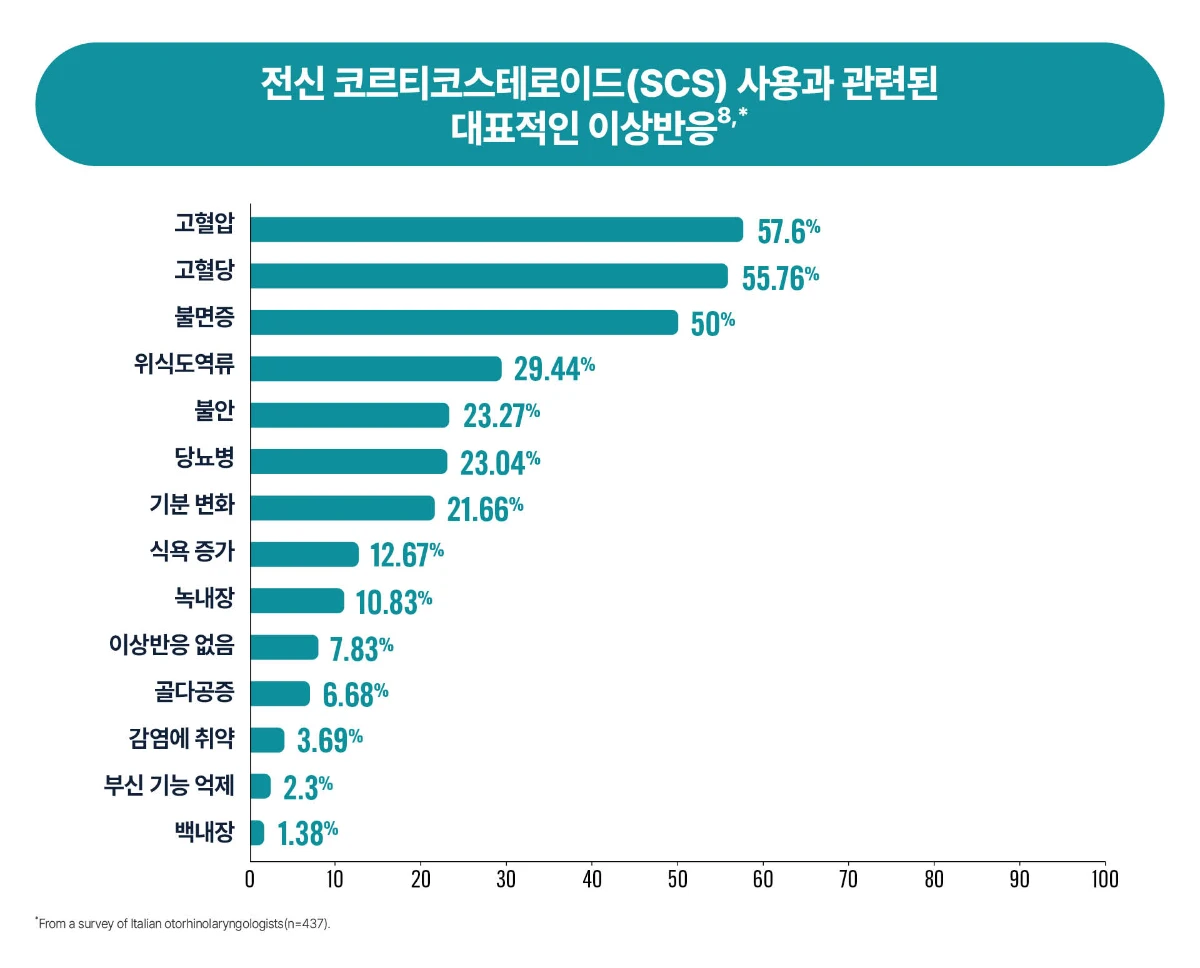
[Study design8] This study conducted an online survey of 437 ENT specialists in Italy to determine current trends in topical and systemic corticosteroid use in patients with CRSwNP. Met remotely to discuss salient aspects in the management of CRSwNP with specific focus on unmet needs regarding the use of local and systemic steroids.
[Study design9] This study aimed to assess HRQoL outcomes (including analyses by disease severity and impact of comorbidities and refractory disease) in CRSwNP patients from a large European database. Data were analysed from the Global Allergy and Asthma European Network (GALEN) Rhinosinusitis Cohort, including sociodemographic data, patient-reported disease severity (visual analogue scale), and scores on the 36-Item ShortForm Health Survey (SF-36) questionnaire.
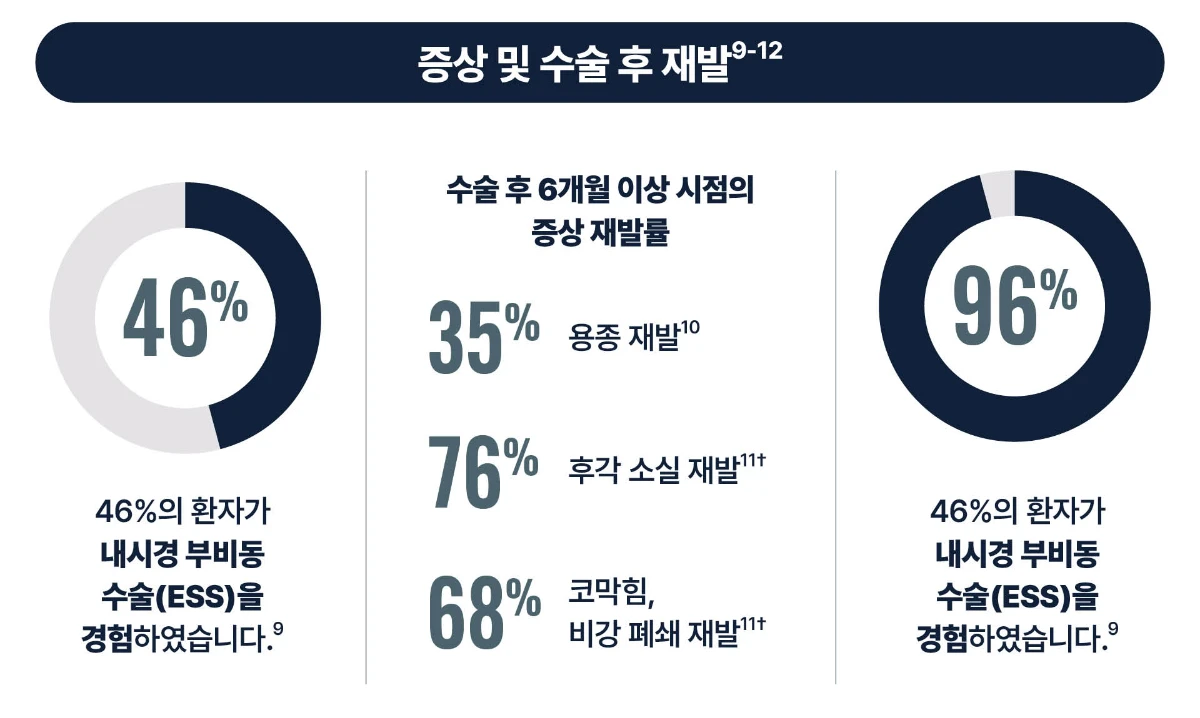
[Study design10] This study objective was to evaluate the prevalence of nasal polyp recurrence up to 18 months after endoscopic sinus surgery (ESS) with congruent continuing medical management. Prospective, multicenter cohort of adult patients undergoing ESS for medically recalcitrant CRSwNP performed between August 2004 and February 2015.
[Study design11] This study was to compare CRS symptoms (nasal obstruction; thick nasal discharge; facial pain/pressure; and reduction or loss of sense of smell.) after sinus surgery with the improvement in CRS symptoms after drug treatment. Cardinal symptoms of CRS were operationalized by 4 questions on the 22-item Sino-Nasal Outcome Test (SNOT-22). Symptom improvement was evaluated in subjects with at least 6-month follow-up.
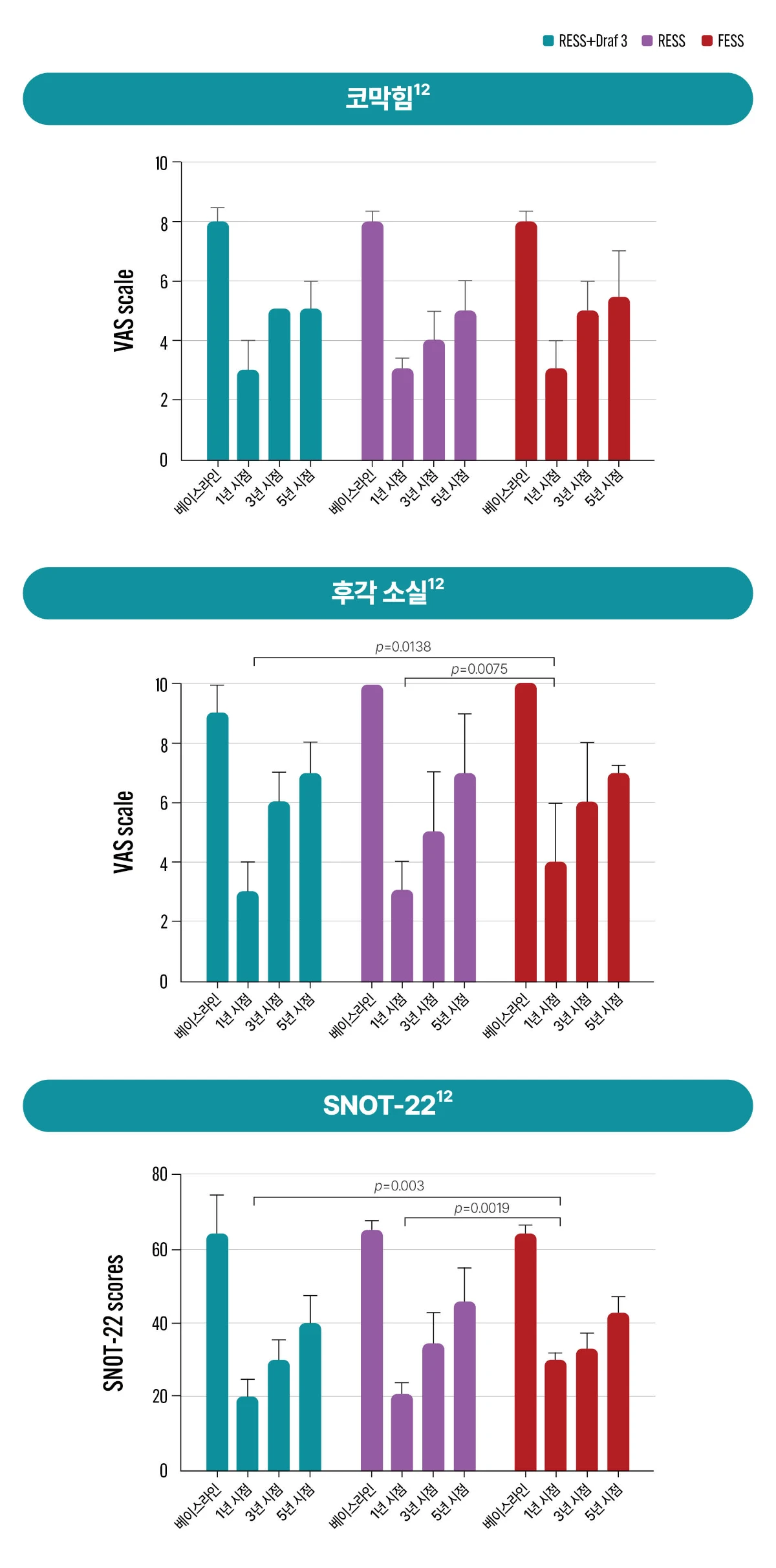
[Study design12] The aim of present study was to investigate the long-term clinical outcomes of extended surgical strategies for patients with recurrent CRSwNP and asthma. Eighty-one patients with CRSwNP and asthma were enrolled in this 5-year prospective study. They were randomly assigned to undergo FESS, radical endoscopic sinus surgery (RESS), or RESS+Draf 3 surgery. Disease severity and clinical outcomes were evaluated using symptoms scoring, endoscopic scoring system, computed tomography staging system, sinus-specific quality of life scores, tissue and peripheral blood eosinophil percentage, and pulmonary function tests. Baseline, 1-year, 3-year, and 5-year follow-up data were compared among the groups.
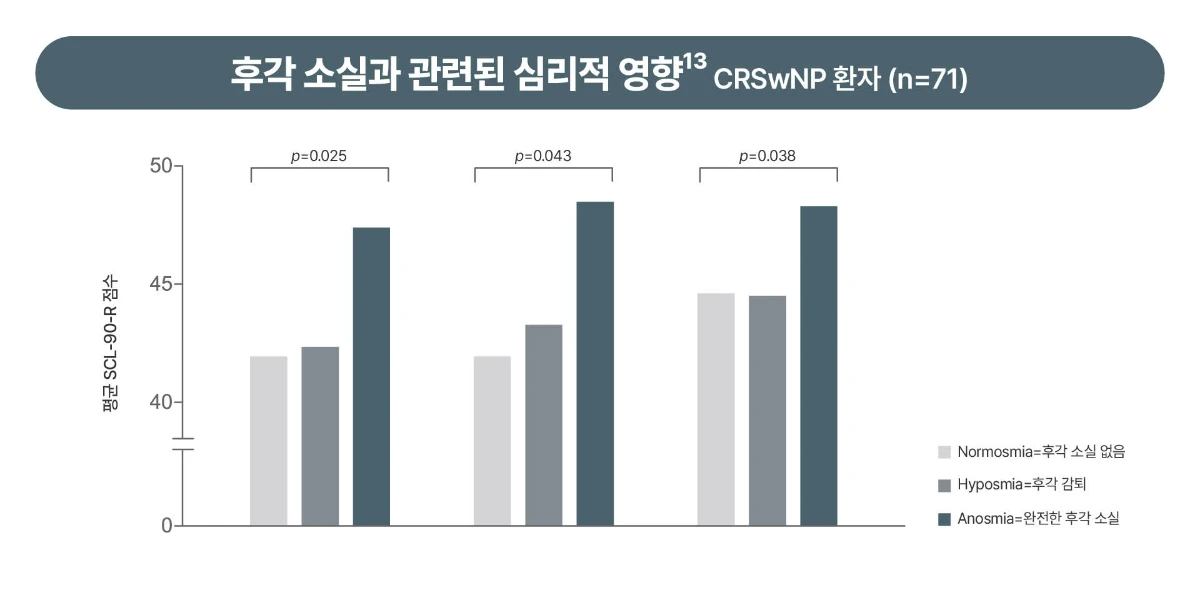
[Study design13] Investigators conducted the impact of olfactory dysfunction on quality of life (QOL) and psychological status in patients with chronic rhinosinusitis with nasal polyps (CRSwNP). A retrospective observational study was conducted from January 2011 to May 2012 with 130 patients with septal deviation (SD) (n = 59) and CRSwNP (n = 71). All patients underwent computed tomography (CT), allergy tests, and sniffin' stick olfactory test. Anosmia was defined by Threshold-Discrimination-Identification (TDI) scores less than 16. QOL and psychological symptoms were assessed with the Sinonasal Outcome Test-20 (SNOT-20) and Symptom Checklist-90-Revised (SCL-90-R).
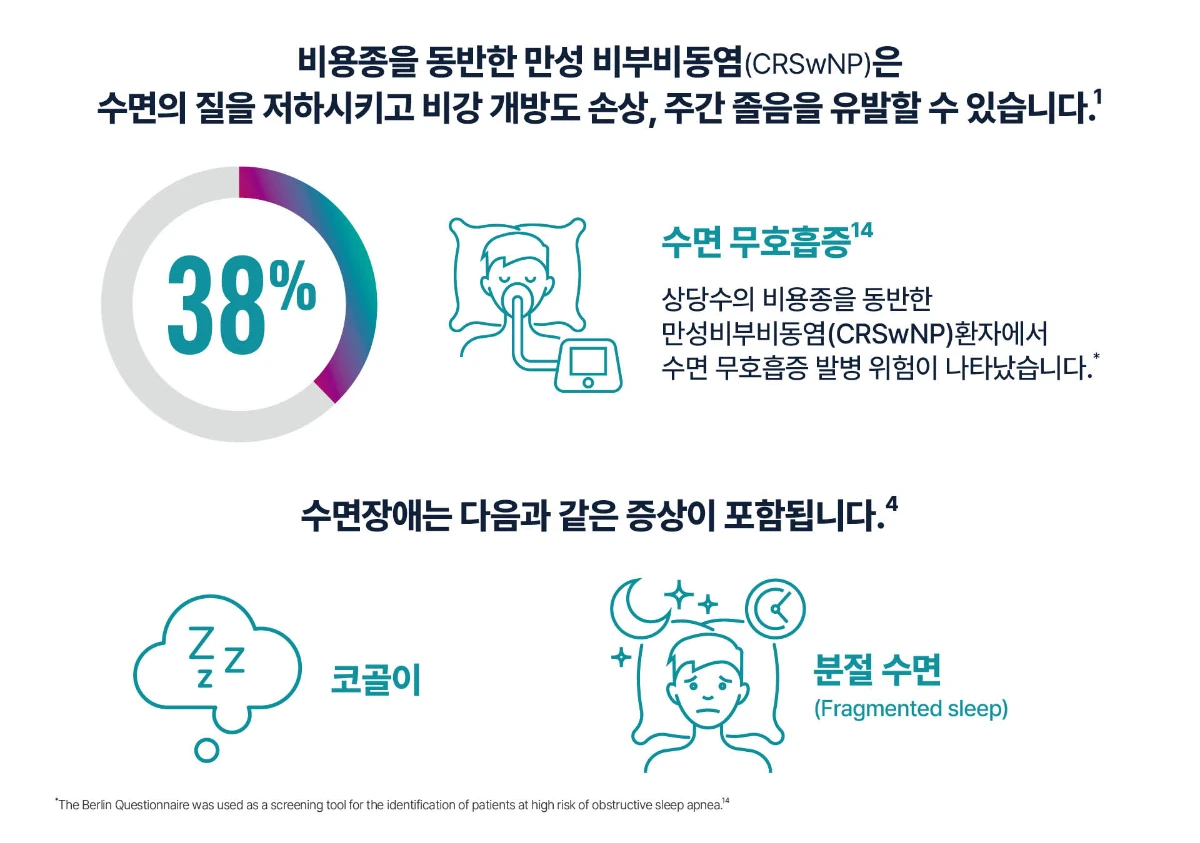
[Study design14] This aim of the study was to investigate sleep quality in patients with CRSwNP before and after endoscopic surgery. Forty-two patients filled out four validated sleep questionnaires and one sino/nasal, disease specific quality of life questionnaire before surgery and three months later. A healthy control group filled out the same questionnaires at baseline and after three months.
AE, adverse event; CRSwNP, chronic rhinosinusitis with nasal polyps; Draf 3, endoscopic modified Lothrop procedure; FESS, functional endoscopic sinus surgery; HRQoL, health-related quality of life; INCS, intranasal corticosteroids; RESS, radical endoscopic sinus surgery; SCL-90-R, Symptom Checklist-90-Revised; SCS, systemic corticosteroids; SNOT-22, 22-item Sinonasal Outcomes Test; VAS, visual analog scale.
※ 약사법 제68조 제6항, 의약품 등의 안전에 관한 규칙 제78조 제1항에 따라 전문의약품의 대중광고는 엄격히 금지되는 바, 본 자료는 광고를 위한 목적으로 제작된 것이 아니며, 질환에 대한 정보 제공을 목적으로 하고 있습니다. 실제 천식 및 만성 비부비동염의 진단과 치료는 전문의를 통한 의학적인 방법과 절차에 따라야 합니다.

