How to use PRALUENT®?
PRALUENT is available as a unique,¥ once-monthly pen for your patients requiring >60% LDL-C reduction2,3
Patient friendly pen2,3
- Hidden needle
- Autoinjector with no button to press
- Self-inject wherever they are
Self-inject quickly3
- Delivers the dose in <20 seconds
PRALUENT can be administered in 4 simple steps2
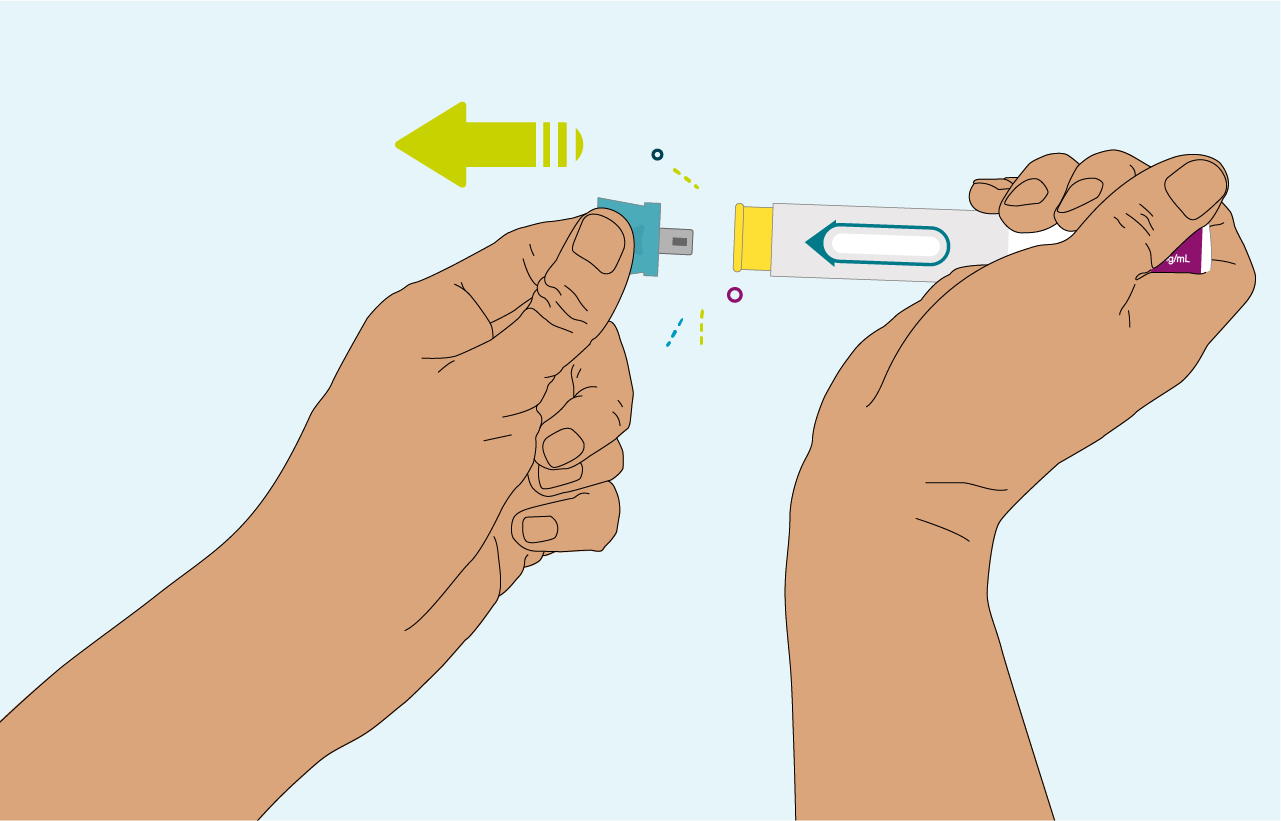
Pull off the blue cap
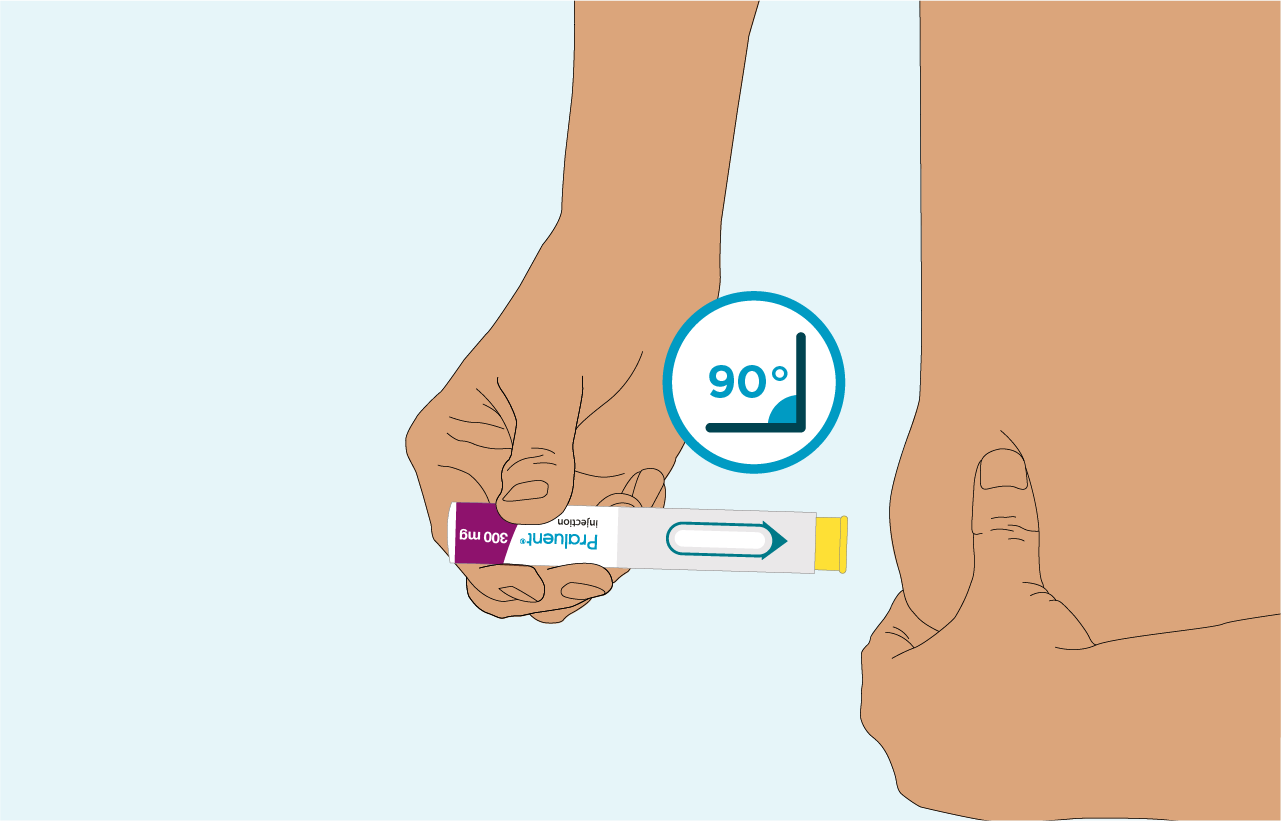
Press the pre-filled pen onto the skin at a 90° angle
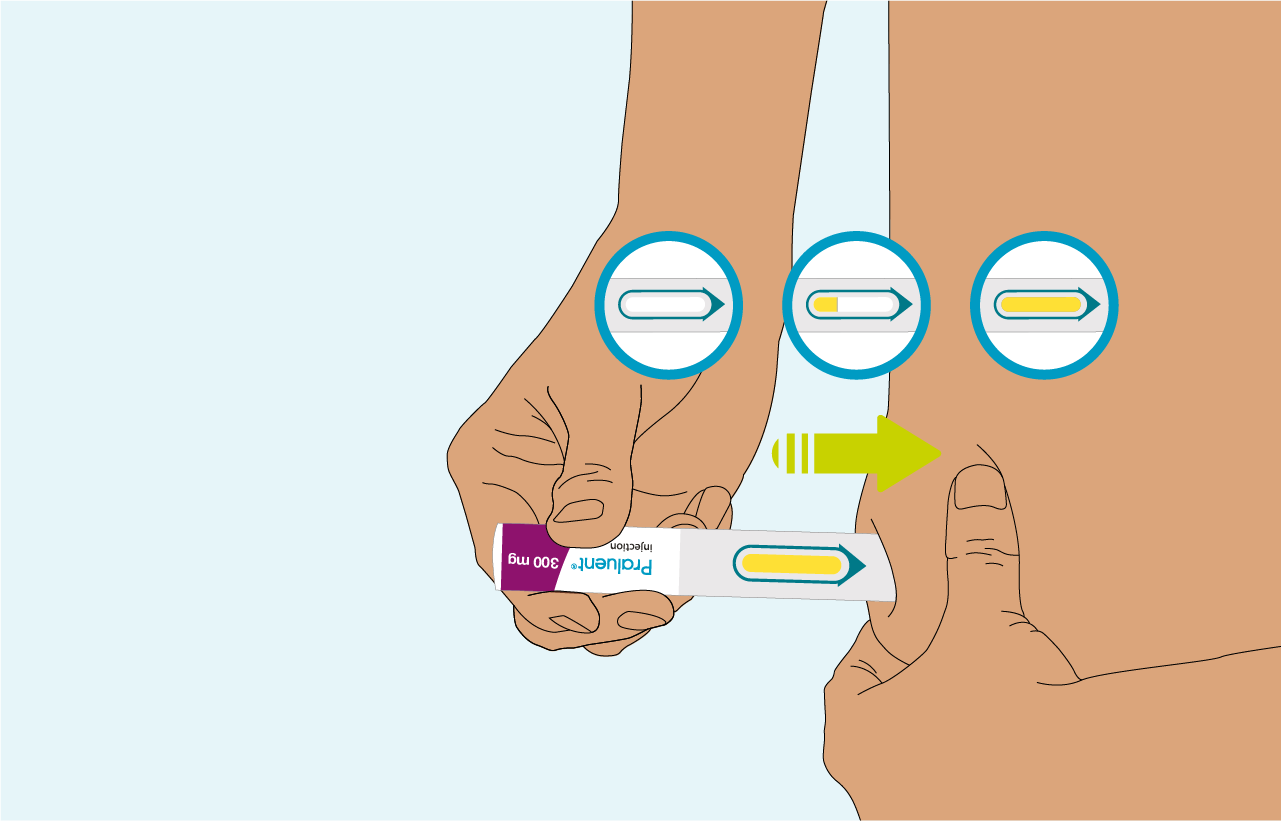
Hold this position untel the inspection window is completely yellow
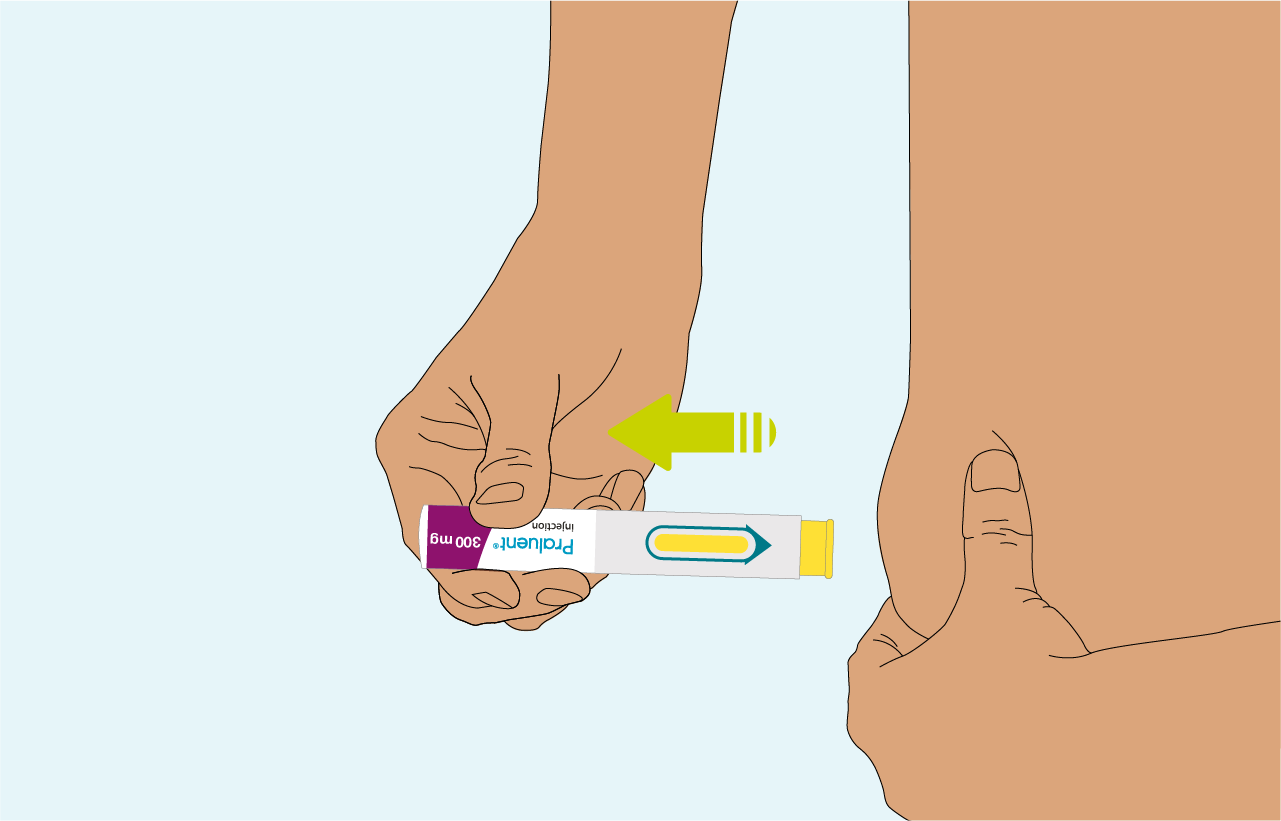
Remove the pre-filled pen from the skin
Primary hypercholesterolaemia and mixed dyslipidaemia2
PRALUENT® is indicated in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, and in paediatric patients 8 years of age and older with heterozygous familial hypercholesterolaemia (HeFH) as an adjunct to diet:2
- in combination with a statin or statin with other lipid lowering therapies in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin or,
- alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated.
Established atherosclerotic cardiovascular disease2
PRALUENT® is indicated in adults with established atherosclerotic cardiovascular disease to reduce cardiovascular risk by lowering LDL-C levels, as an adjunct to correction of other risk factors:
- in combination with the maximum tolerated dose of a statin with or without other lipid-lowering therapies or,
- alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated.
Adults2
Prior to initiating PRALUENT® secondary causes of hyperlipidaemia or mixed dyslipidaemia (e.g., nephrotic syndrome, hypothyroidism) should be excluded.
The usual starting dose for alirocumab is 75 mg administered subcutaneously once every 2 weeks. Patients requiring larger LDL-C reduction (>60%) may be started on 150 mg once every 2 weeks, or 300 mg once every 4 weeks (monthly), administered subcutaneously.
The dose of PRALUENT® can be individualised based on patient characteristics such as baseline LDL-C level, goal of therapy, and response. Lipid levels can be assessed 4 to 8 weeks after treatment initiation or titration, and dose adjusted accordingly (up-titration or down-titration).
If additional LDL-C reduction is needed in patients treated with 75 mg once every 2 weeks or 300 mg once every 4 weeks (monthly), the dosage may be adjusted to the maximum dosage of 150 mg once every 2 weeks.
HeFH in paediatric patients 8 years of age and older:2
| Body weight of patients | Recommended dose | Recommended dose if additional LDL-C reduction is needed* |
| Less than 50 kg | 150 mg once every 4 weeks | 75 mg once every 2 weeks |
| 50 kg or more | 300 mg once every 4 weeks | 150 mg once every 2 weeks |
Adapted from PRALUENT® Summary of Product Characteristics. 2023.2
* Lipid levels can be assessed 8 weeks after treatment intiation or titration and dose adjusted accordingly.2
If a dose is missed, the dose should be administered as soon as possible and thereafter, dosing should be resumed on the original schedule.
*62.7% LDL-C reduction compared to placebo at 4 months in ODYSSEY OUTCOMES trial.1
†MACE: primary composite endpoint of CHD death, non-fatal myocardial infarction, fatal and non-fatal ischaemic stroke, or unstable angina requiring hospitalisation. HR 0.85 (95% CI 0.78, 0.93), P=0.0003.1,2
‡With only nominal statistical significance by hierarchical testing; HR 0.85 (95% Cl 0.73, 0.98), P=0.0261.1,2
¥For patients requiring LDL-C reduction >60%, PRALUENT is the only PCSK9i with once-monthly single injection in a pre-filled pen.2
CI = confidence interval; HeFH = Heterozygous Familial Hypercholesterolaemia; HR = hazard ratio; LDL-C = low-density lipoprotein cholesterol; MACE = major adverse cardiovascular event; PCSK9i = proprotein convertase subtilisin/kexin type inhibitor.
-
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107.
-
PRALUENT (alirocumab) Summary of Product Characteristics. Paris, France: sanofi-aventis groupe; December 2023.
-
Frias JP, Koren MJ, Loizeau V, et al. The SYDNEY device study: a multicenter, randomized open-label usability study of a 2-mL alirocumab autoinjector device. Clin Ther. 2020;42(1):94–107.
More information
Click a link below to learn more about PRALUENT
What is PRALUENT?
Click here to learn about how PRALUENT works and who it is for
Why choose PRALUENT?
Click here to learn more about the efficacy and safety profile of PRALUENT