Overview of cGvHD
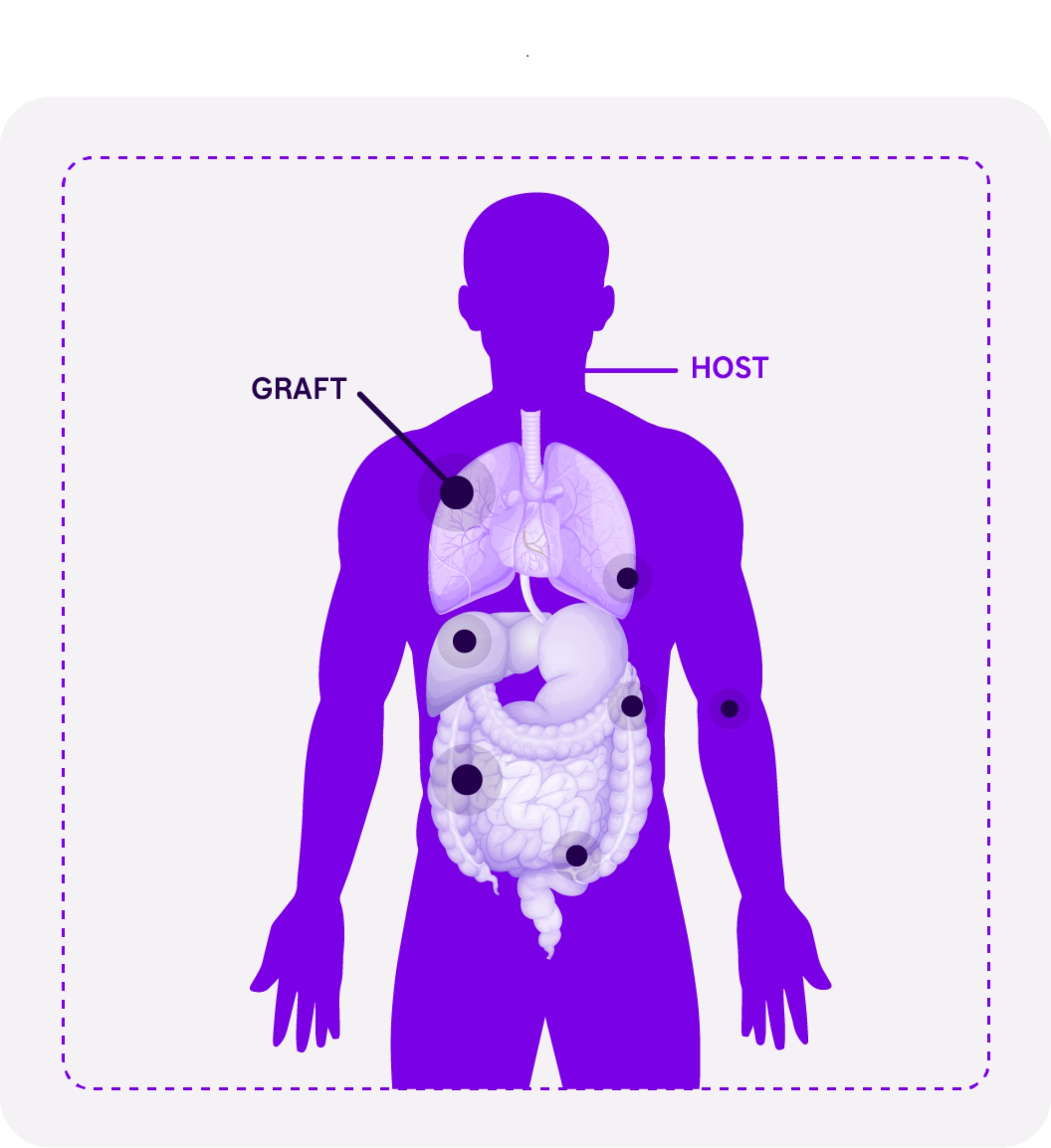
GvHD leads to serious and potentially life-threatening complications from both inflammatory and fibrotic processes.⁴
Overview of GvHD
- GvHD is characterized into aGvHD or cGvHD based on clinical features.5
- GvHD is a common systemic complication that can occur post alloHCT.6-8
- Grafted immune cells attack the host’s (or recipient’s) cells because they are recognized as foreign,6-8 causing an uncontrolled inflammatory response.8
Allo-HSCT recipients can develop one, both or neither type of GvHD.8,9
Difference between aGvHD vs. cGvHD
- The key difference between aGvHD and cGvHD stems from the fact that aGvHD is primarily an inflammatory reaction11 whereas cGvHD is a more complex condition that involves both inflammatory and fibrotic processes.7
- aGvHD is an immune reaction that is primarily mediated by donor T cells.11
- cGvHD involves donor T-cells and other donor immune cells (beyond B-cells, also macrophages/monocytes).12
Risk factors for aGvHD
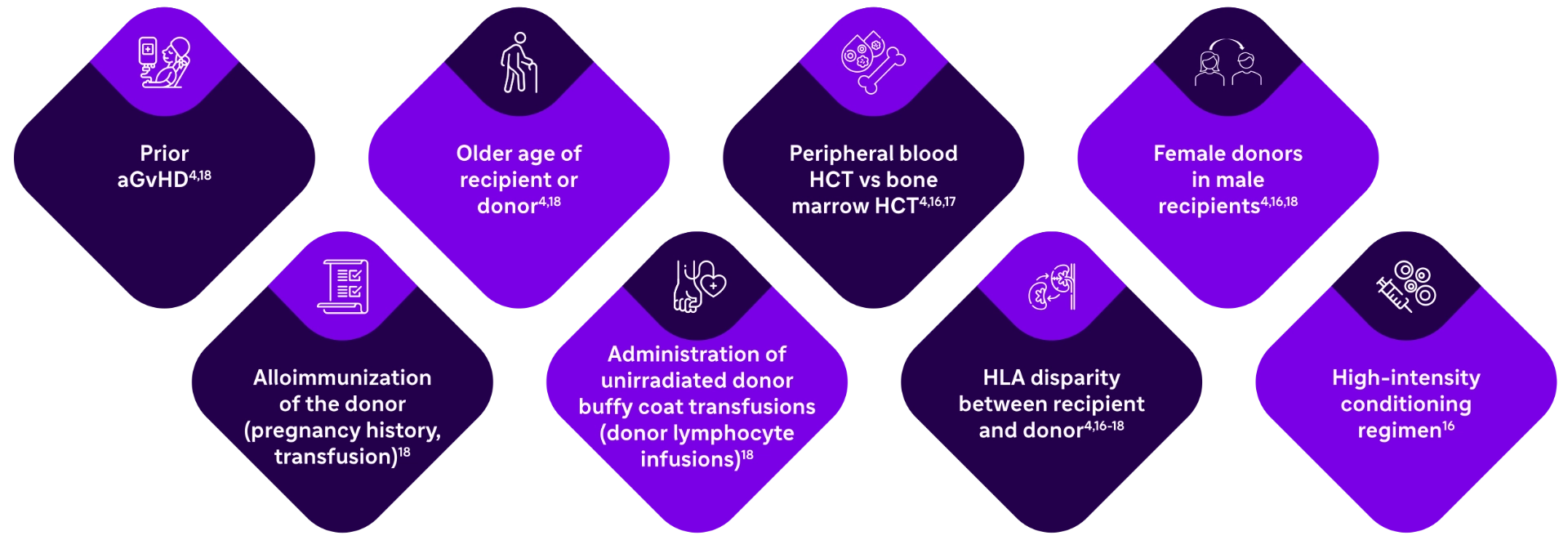
Common risk factors for patients developing aGvHD after receiving an alloHCT are shown here.
Recipients of an unrelated donor type alloHCT have a 1.4 times greater risk of developing aGvHD compared with recipients of a sibling donor type alloHCT. In contrast, graft source (peripheral blood vs bone marrow) does not appear to affect a patient’s risk of developing aGvHD.13
| Risk Factors for cGvHD |
|
Although many risk factors overlap between aGvHD and cGvHD,10 one key difference is that a prior history of aGvHD is a risk factor for developing cGvHD.15 An estimated 52% of patients progress from aGvHD to cGvHD, 9% are de novo cases, and 39% show quiescent onset.14 Age ≥18 years increases risk: 5-fold with marrow/cord graft, 10-fold with peripheral blood graft.1 |
Classifications of GvHD
The classification of GvHD into aGvHD or cGvHD is no longer based on time post alloHCT. Instead, it is based on clinical features defined in the 2014 NIH cGvHD Consensus Criteria.10
What does cGvHD look like?
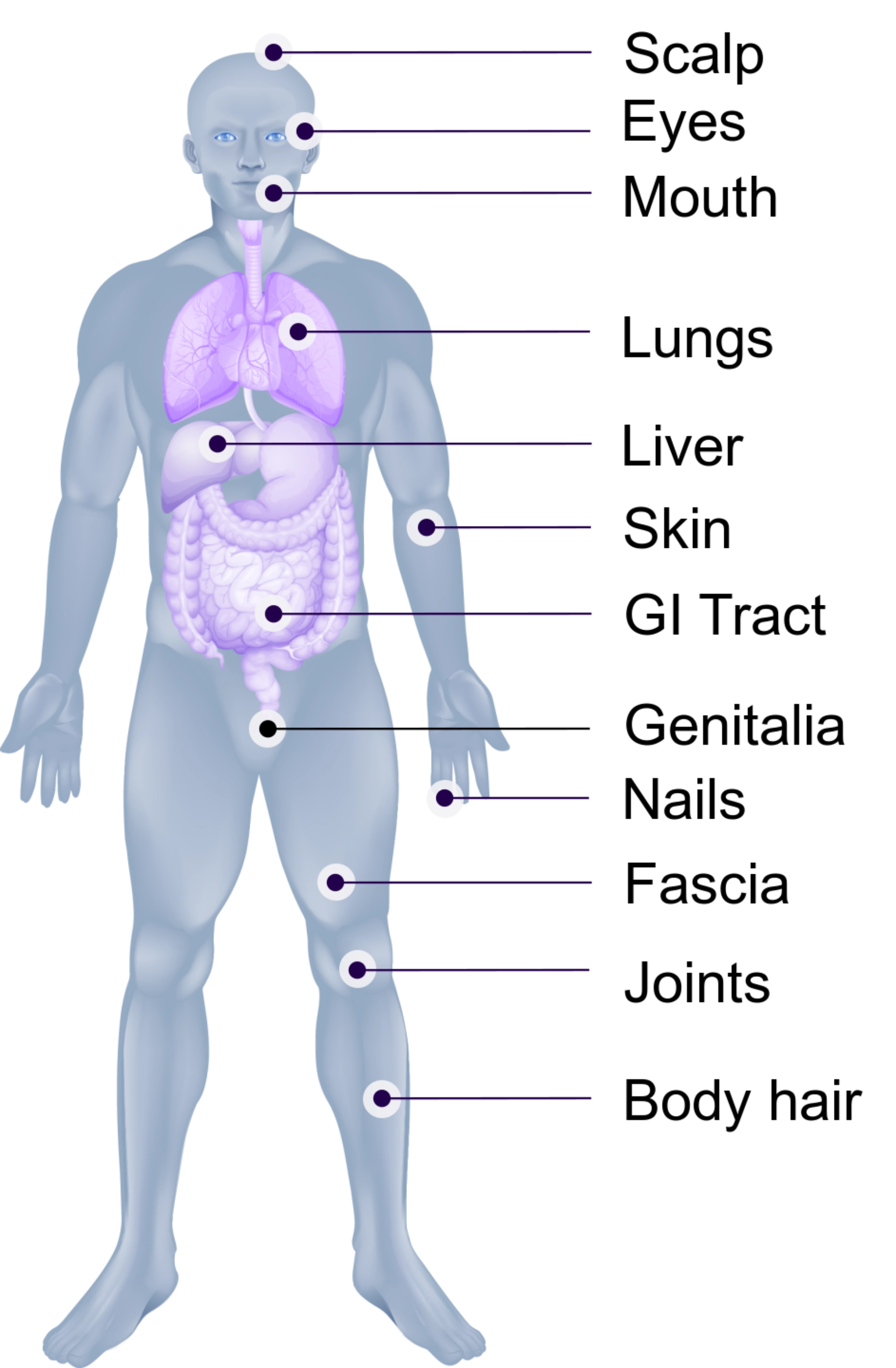
Unlike aGvHD, cGvHD has more heterogeneous organ involvement and variable disease manifestations. Commonly affected areas include the skin, nails, scalp and body hair, mouth, eyes, genitalia, GI tract, liver, lungs, fascia, and joints.
Chronic GvHD is characterized by fibrosis and inflammation. Fibrosis is a major contributor to life-threatening complications and disability.
Onset of cGvHD in many patients presents as a skin rash, mouth sensitivity or dryness, or dry/irritated eyes. Symptom progression in these organs as skin sclerosis, oral ulcers, severe dry eye, and lung involvement such as bronchiolitis obliterans syndrome (BOS) become very difficult to treat and control.
aGvHD, acute graft-versus-host disease; alloHCT, allogeneic hematopoietic cell transplant; cGvHD, chronic graft-versus-host disease; GvHD, graft-versus-host disease; HCT, hematopoietic stem cell transplant; HLA, human leukocyte antigen; vs, versus.
- Justiz Vaillant AA, et al. Graft versus host disease. In: StatPearls. StatPearls Publishing; 2020. Updated September 19, 2020. Accessed August 20, 2020. https://www.ncbi.nlm.nih.gov/books/NBK538235;
- Henden AS, Hill GR. J Immunol. 2015;194(10):4604-4612. doi:10.4049/jimmunol.1500117;
- Ramachandran V, et al. Dermatol Clin. 2019;37(4):569-582. doi:10.1016/j.det.2019.05.014;
- Zeiser R, Blazar BR. N Engl J Med. 2017;377(26):2565-2579. doi:10.1056/NEJMra1703472;
- Kim D et al. Transplantation Proceedings 2024;409-415.;
- Justiz Vaillant AA et al. In: StatPearls. StatPearls Publishing; 2020. Updated May 1, 2022. Accessed July 18, 2023. https://www.ncbi.nlm.nih.gov/books/NBK538235;
- . Henden AS, Hill GR. J Immunol. 2015;194(10):4604-4612. doi:10.4049/jimmunol.1500117;
- Ramachandran V et al. Dermatol Clin. 2019;37(4):569-582. doi:10.1016/j.det.2019.05.014;
- Lee SJ. Blood. 2017;129(1):30-37. doi:10.1182/blood-2016-07-686642;
- Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001;
- Ferrara JLM et al. Lancet. 2009;373(9674):1550-1561. doi:10.1016/S0140-6736(09)60237-3;
- Mankarious M et al. Front Immunol. 2020;11:81. doi:10.3389/fimmu.2020.00081;
- Lee SE et al. Bone Marrow Transplant. 2013;48(4):587-592;
- Mawardi H et al. Oral Dis. 2019;25(4):931-948;
- Lazaryan A et al. Biol Blood Marrow Transplant. 2016;22(1):134-140;
- Cooke KR et al. Biol Blood Marrow Transplant. 2017;23(2):211-234;
- Hymes SR et al. J Am Acad Dermatol. 2012;66(4):515.e1-515.e18;
- Stewart BL et al. Blood. 2004;104(12):3501-3506;
- Lee SJ. Blood. 2017;129(1):30-37. doi:10.1182/blood-2016-07-686642;
- MacDonald KPA et al. Blood. 2017;129(1):13-21. doi:10.1182/blood-2016-06-686618;
- Kitko CL et al. Biol Blood Marrow Transplant.2012;18(suppl 1):S46-S52. doi:10.1016/j.bbmt.2011.10.021;
- Flowers MED, Martin PJ. Blood. 2015;125(4):606-615. doi:10.1182/blood-2014-08-551994.
- Malard F et al. Nat Rev Dis Primers. 2023;9(1):27.




