{
event: "article_read",
name: `Guidelines`,
author: `Sanofi`,
tags: `Transplant`,
publication_date: `2025-08-21`,
interaction_type: "content"
}
Guidelines
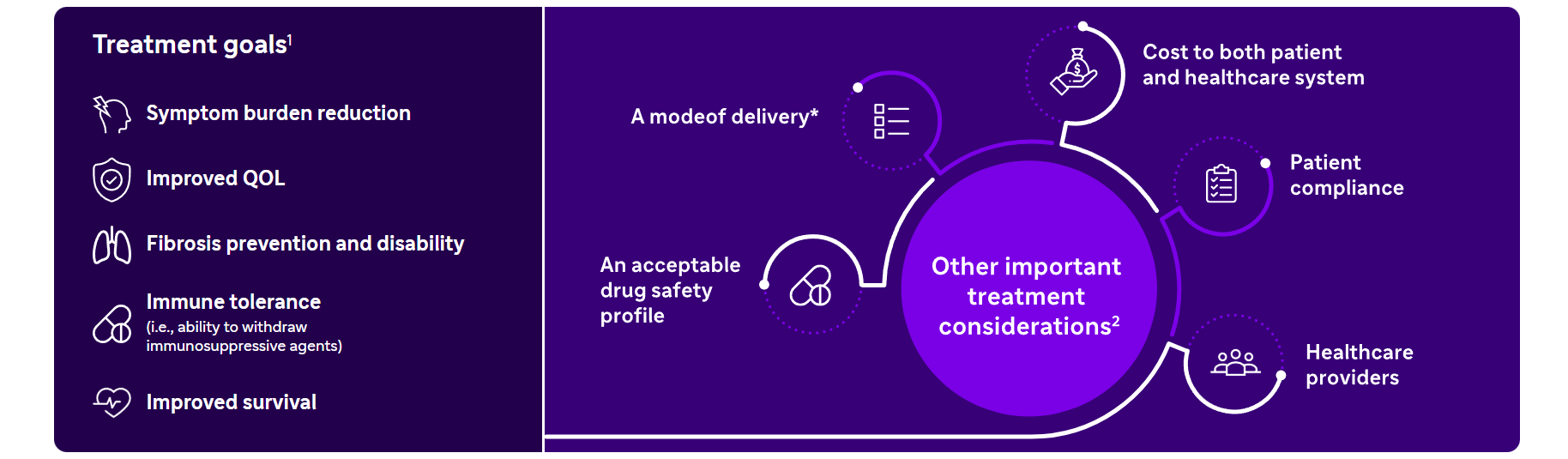
Aim of cGvHD treatment and general considerations
NCCN guidelines for the management of cGvHD3
*central line access, IV infusion, injection, pill that is convenient for patients.
BD, twice daily; cGvHD, chronic-graft-versus host disease; CR, complete response; CS, corticosteroids; CNIs, calcineurin inhibitors; ECP, Electrocorproeal photopheresis; FFS, failure-free survival; MMF, Mycophenolic acid; NR, not registered; OD, once daily; ORR, overall response rate; PR, partial response; QoL, quality of life; SR, steroid refractory; SOC, standard of care; URTI, upper respiratory tract infection; NCCN, National Comprehensive Cancer Network; EBMT, European Society for Blood and Marrow Transplantation.
- Hamilton BK. Hematology Am Soc Hematol Educ Program. 2021;2021(1):648-654.;
- Malard F, et al. Am J Hematol. 2023;98(10):1637-1644;
- Hematopoietic Cell Transplantation, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology;
- Flowers MED, et al. Blood. 2015;125(4):606-615.;
- Dignan FL, et al. British journal of haematology. 2012;158(1):46-61.;
- Zeiser R. J Clin Oncol. 2023;41(10):1820-1824.;. Hamilton BK. Hematology Am Soc Hematol Educ Program. 2021;2021(1):648-654.
- Kim D.D.H et al. Curr Oncol. 2024;31:1426-1444.;
- Milkos D et al., Blood. 2017;130:2243-2250.;
- Cutler C et al. Blood. 2021;138:2278-2289.;
- Zeiser R et al. N Engl J Med. 2021;385:228-238.
MAT-BE-2500625, v1.0, May 2025