Actors
Dendritic CellDendritic cells are the pivot between innate immunity and adaptive immunity, the only cells capable of cross-presentation and capable of activating naive T cells.
As such, they are the pivot between innate and adaptive immunity, an essential actor of the immune reaction. They are the leader of professional antigen-presenting cells.
|
|
To go further:
Attention to “false friends”: do not confuse dendritic cells and follicular dendritic cells
Follicular dendritic cells have this name because they are cells with large cytoplasmic extensions, located within lymphoid follicles.
Although they have the same name as dendritic cells, their role and characteristics are very different.
- They are not the product of haematopoiesis, but come from stromal cells.
- They do not present antigen for the activation of T cells.
On the other hand, they interact very closely with B cells (they are involved in particular in their maturation and differentiation) and therefore play an important role in the T-dependent humoral immune response.
| HAEMATOPOIESIS / CYTOLOGY |
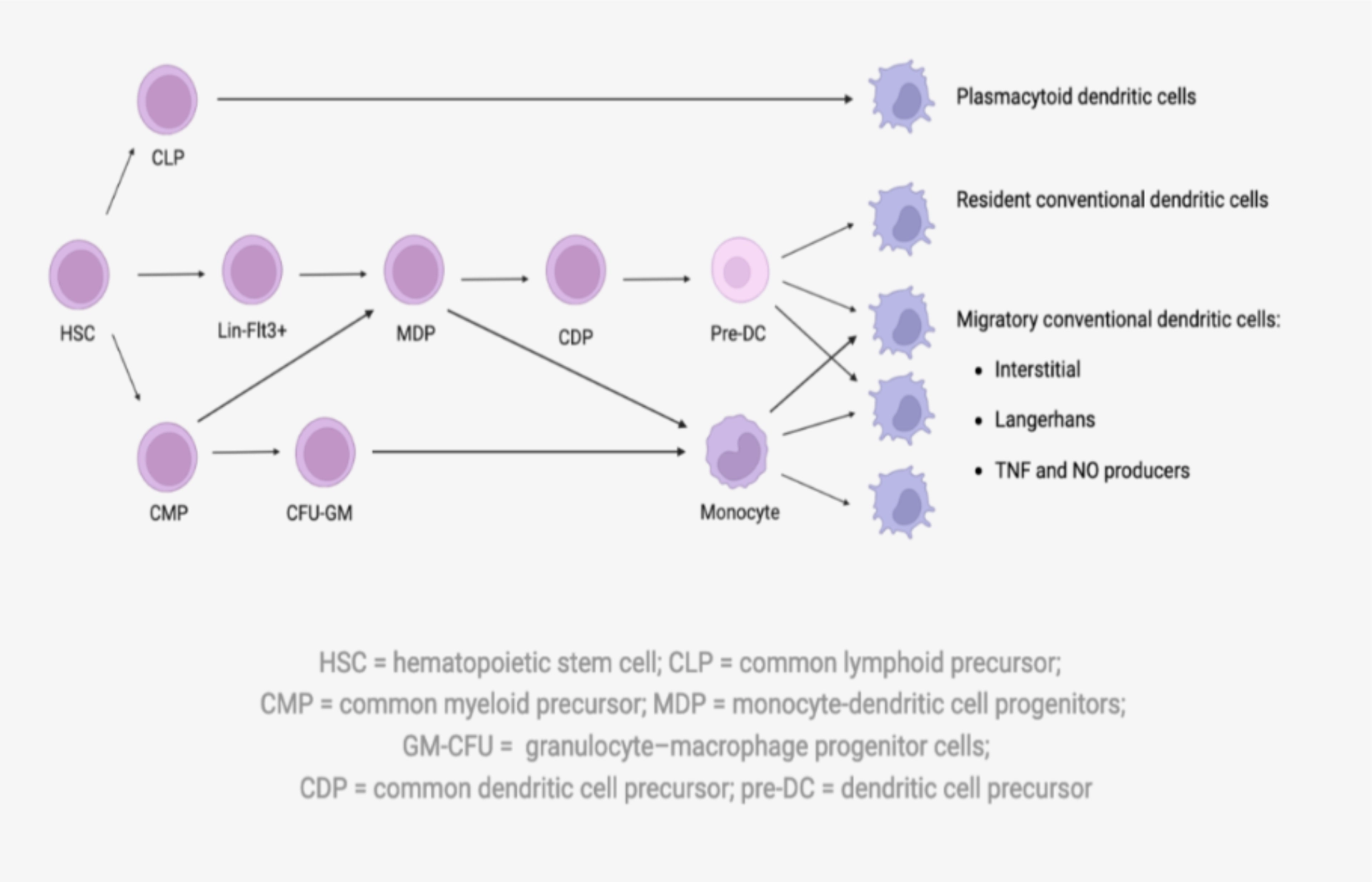
| Dendritic cells are very heterogeneous in origin and purpose. Although most have haematopoietic progenitors, they are virtually absent from the bloodstream because they reside primarily in secondary lymphoid organs. We distinguish between: |
Myeloid dendritic cells (so-called conventional)
1. Migratory
- They are the archetype of dendritic cells. Naïve, they reside in tissues with a cytoplasm typically displaying dendrites, but a morphology which will vary from one tissue to another. They are then sensitive to danger signals and capable of phagocytising antigens before migrating to secondary lymphoid organs, where they present the antigen to naive T cells.
2. Residents of secondary lymphoid organs
- ological and cytometric characteristics as the previous ones, but are sedentary in the secondary lymphoid organs where they collect and present antigens.
| Plasmacytoid dendritic cells |
| Plasmacytoid dendritic cells are circulating, round cells in their basal state. After activation, they acquire a phenotype of conventional dendritic cells, and can therefore present the antigen (most often viral) then produce a large quantity of pro-inflammatory cytokines. |
To go further: Dendritic cell ontogeny
FUNCTIONS
They have distinct functions depending on their state of maturation, which correlates with their location.
Immature dendritic cells ensure antigen uptake
Dendritic cells are then sentinel cells, which:
- are recruited by cytokines and chemokines produced by inflammatory tissues.
- recognise danger signals (MAMP and DAMP) by innate immunity receptors called PRRs, intracellular or expressed on the surface of the cell.
Intracellular receptors are useful if the cell has already phagocytosed the antigen!
3. capture the antigen by three different endocytosis mechanisms:
Once the antigen has been captured, the maturation process is initiated.
Dendritic cells mediate antigen presentation to T cells
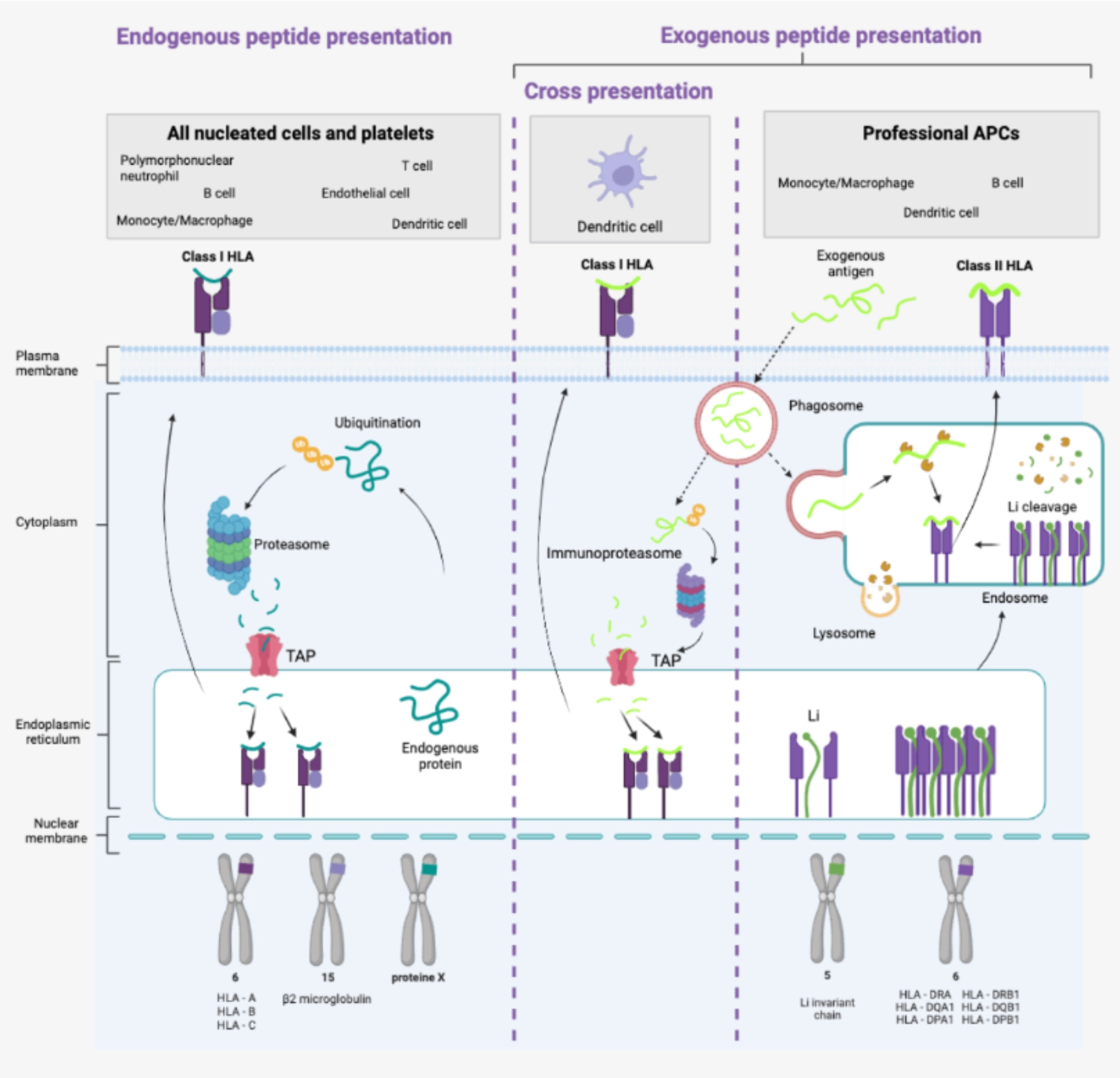
- Dendritic cells are specialised in antigen presentation.
- They have Class I HLA molecules in the same way as all nucleated cells and platelets. They have the particularity – shared with all so-called professional antigen-presenting cells – of possessing Class II HLA molecules allowing the presentation of exogenous antigens.
|
Dendritic cells are the only cells capable of cross-presentation, i.e. of presenting exogenous peptides via Class I HLA molecules. |
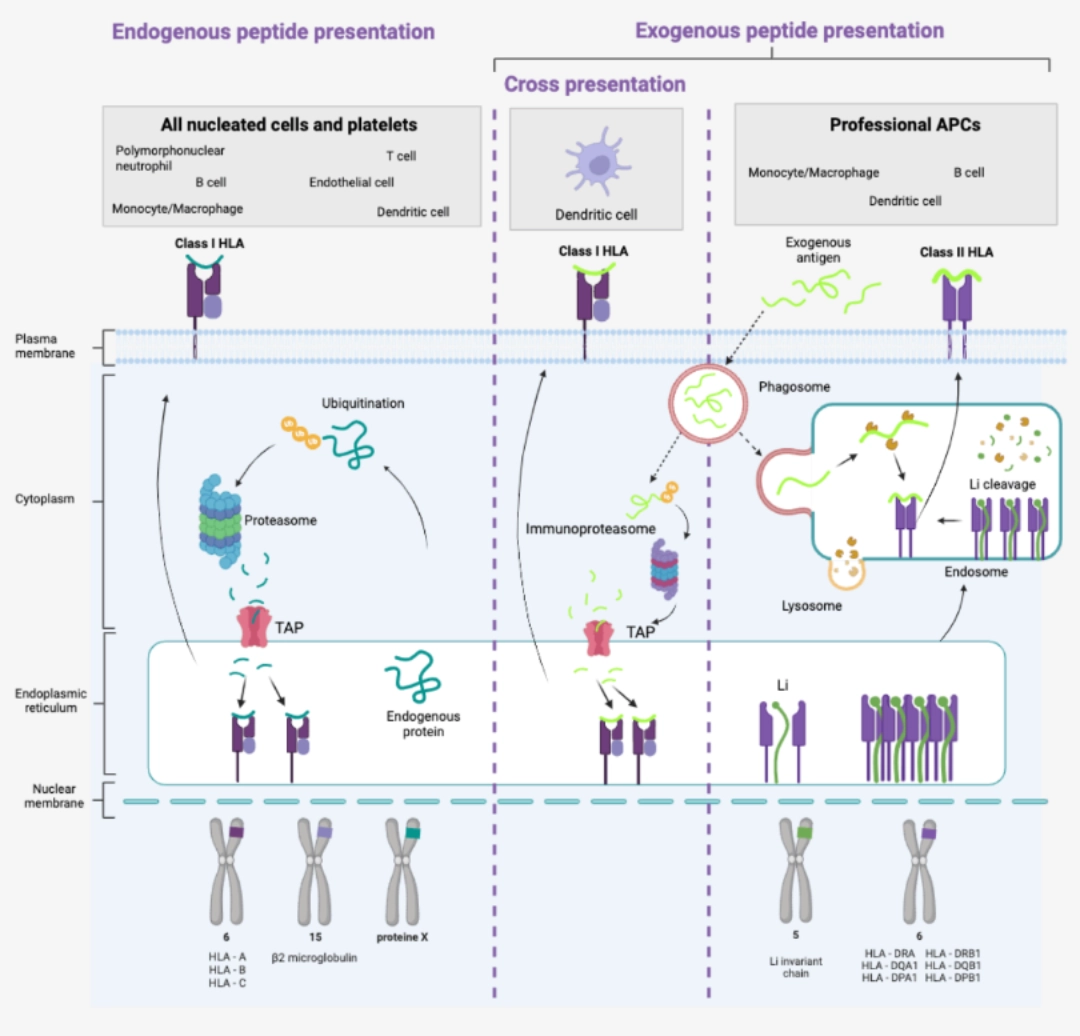
|
Mature dendritic cells mediate activation of naive T cells
Depending on the cellular interactions and the cytokine environment at the inflammatory site, the dendritic cell acquires a new phenotype capable of activating naive T cells and inducing different types of T response. This new phenotype is characterised by very high expression. This new phenotype is characterised by very high expression.
- of HLA molecules to present the antigen to T cells,
- of co-stimulation molecules (CD80, CD86, CD40) to enable the activation of T cells,
- and of CCR7 leading to the migration of dendritic cells towards the lymph nodes (place of concentration of naive T cells).
Mature dendritic cells secrete cytokines which will direct the T-cell response
- In addition to antigen recognition, T cell activation requires cytokine signals to be complete (see T cell activation)
What needs to be remembered
B cells are effector cells of adaptive immunity, particularly humoral, because plasma cells are the only cells capable of producing antibodies. These antibodies are of major importance in the context of transplantation, which is why B cells are the target of immunosuppressants. There are also techniques to eliminate or render non-functional antibodies.
To go further
Cross-presentation is also useful outside of transplantation, to induce cellular immunity against intracellular pathogens that do not usually infect dendritic cells or against tumour cells.
The monocyte differentiates into a macrophage.
Monocytes are a group of heterogeneous cells that migrate into tissues to differentiate into macrophages. They are part of the phagocytic cell family including dendritic cells and polymorphonuclear neutrophils.
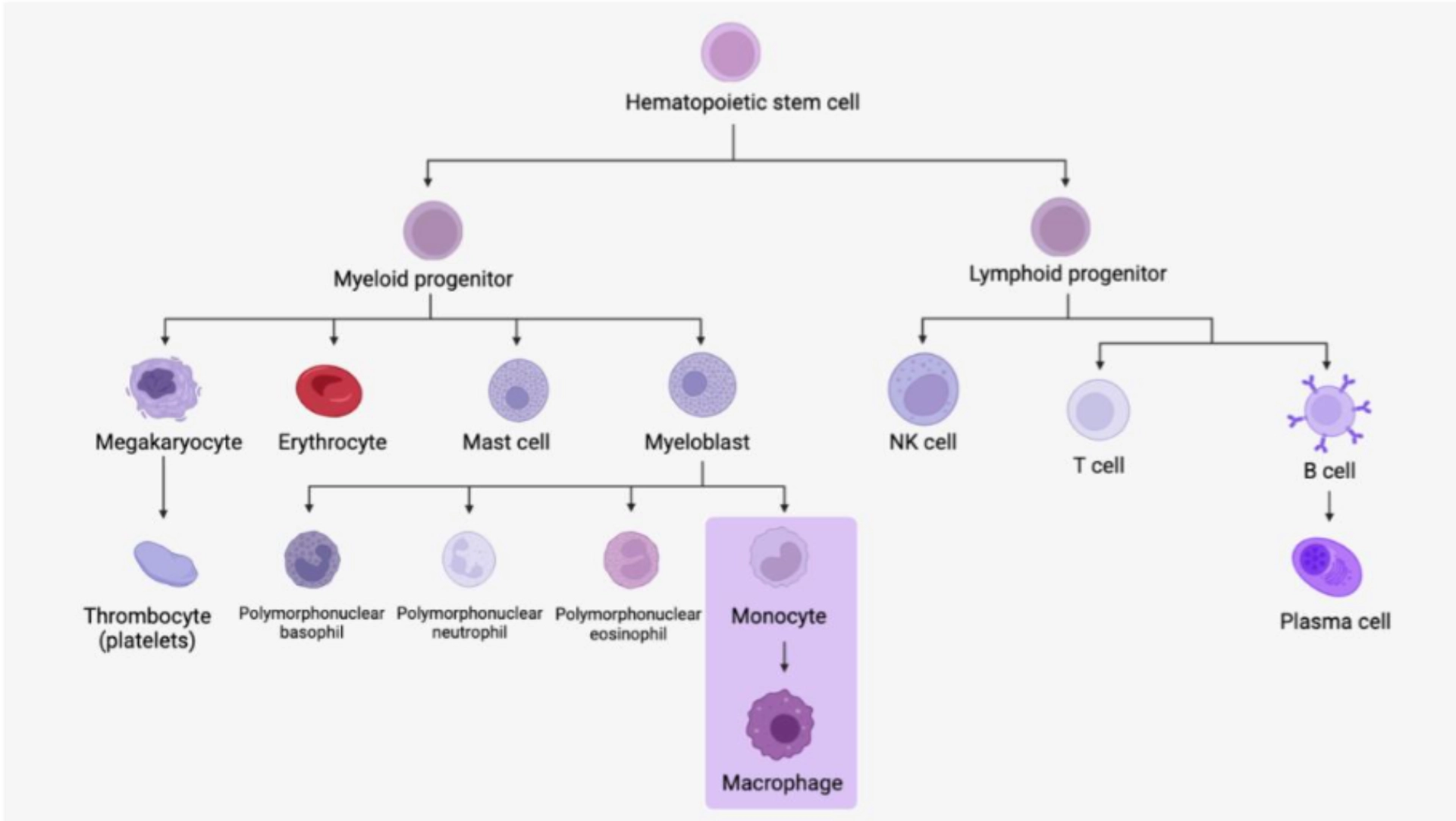
Monocytes
Monocytes represent 5 to 10% of circulating leukocytes. They are large rounded cells whose nucleus most often has a bean-shaped appearance with a notch, a flaky gray cytoplasm with fine granulations and sometimes visible cytoplasmic vacuoles.
Macrophages
Monocytes that pass into tissues are called macrophages. Their cytoplasm grows, and the nucleus becomes eccentric within the cytoplasm which contains more numerous vacuoles. The precise morphology and naming then depend on the tissue where they have penetrated (for example in the bone = osteoclasts, in the central nervous system = microglial cells, in the lungs = alveolar macrophages, etc.).
We can distinguish two types of macrophages:
1. Residents
-
They have functions of regulation, repair, and regeneration of the tissue.
2. Inflammatory
-
They participate in the innate immune response through phagocytosis and antigen presentation.
To go further:
There are also subcategories of monocytes that are not detailed here.
FUNCTIONSPhagocytosisThis is the main function of macrophages. The latter can be facilitated by opsonization, that is to say, the recognition of immunoglobulins or C3b fragments of the complement system fixed on the antigen or on the cell to be phagocytized (in particular foreign cell) by receptors expressed on the surface of the macrophage, respectively the receptors for constant fragments of immunoglobulins and the C3b receptors. |
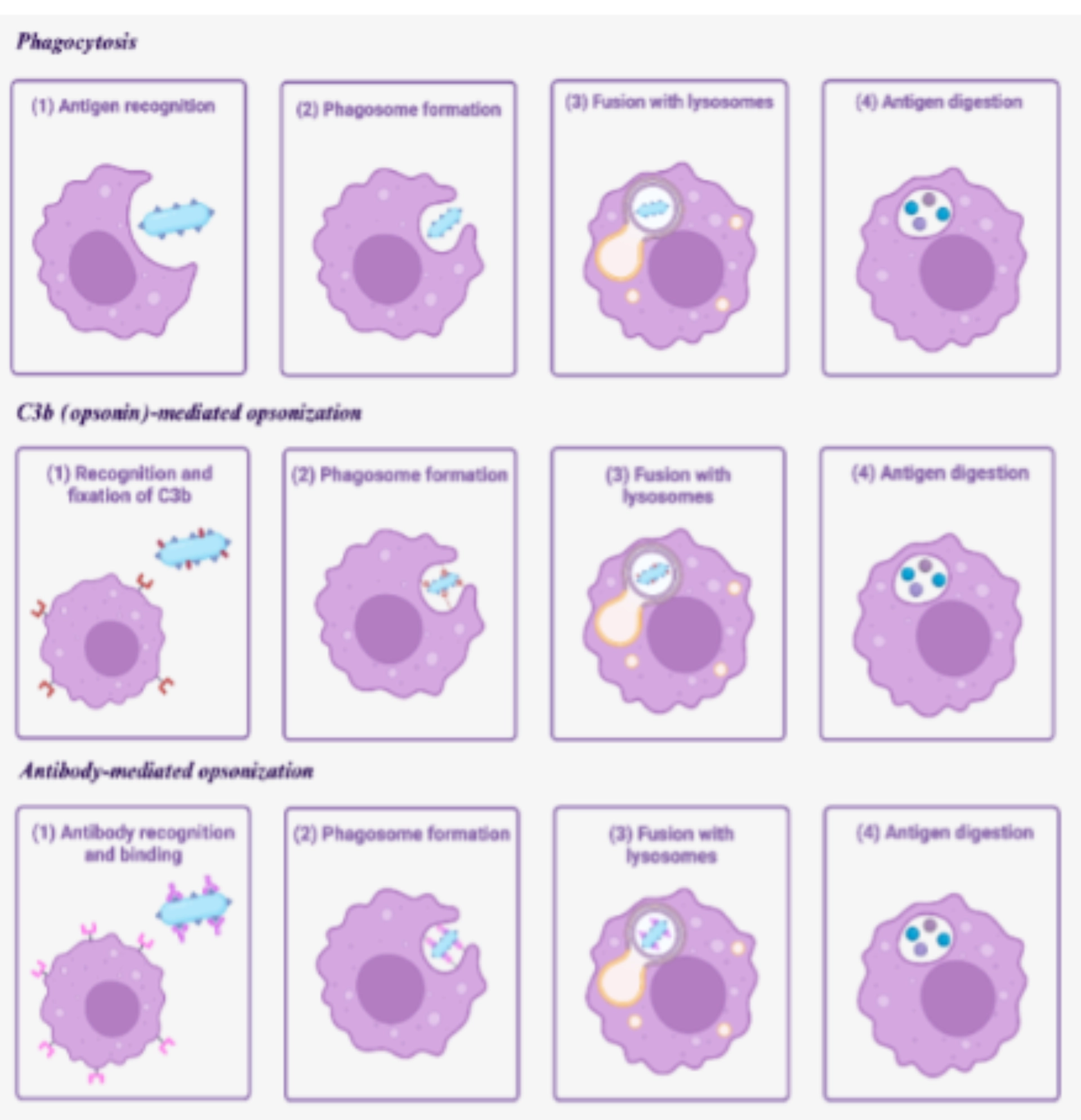 |
Antigen presentationMacrophages possess the necessary mechanisms for antigen presentation of endogenous peptides peptides in the same way as all nucleated cells and platelets. They have the particularity – shared with all so-called professional antigen-presenting cells – of also possessing Class II HLA molecules allowing the presentation of exogenous antigens.
Unlike dendritic cells, however, they are not capable of cross-presentation (no cross-presentation). |
 |
Cytokine synthesis
- Monocytes and macrophages secrete numerous cytokines , particularly pro-inflammatory ones (IL-1, IL-6 and TNF-α).
Homeostatic functions
- Macrophages often have a role in the regeneration and renewal of the tissue in which they reside.
What needs to be remembered
B cells are effector cells of adaptive immunity, particularly humoral, because plasma cells are the only cells capable of producing antibodies. These antibodies are of major importance in the context of transplantation, which is why B cells are the target of immunosuppressants. There are also techniques to eliminate or render non-functional antibodies.
B CELL AND PLASMA CELLB cells and plasma cells are the actors in humoral immunity, that is to say, mediated by antibodies. B cells are capable of recognising a native antigen.
B cells are responsible for humoral immunity, and as such are one of the effector pillars of adaptive immunity in the same way as T cells. They are also professional antigen-presenting cells. |
 |
HAEMATOPOIESIS / CYTOLOGY
| B cells |
| Cytological characteristics and the main principles of B cell ontogeny are described here. |
| Plasma cells |
|
Some B cells become plasma cells. In the physiological state, they are found in the secondary lymphoid organs where they are generated or in the bone marrow where they return to nest (see B cell activation). It is a large oval cell whose nucleus is eccentric within a large basophilic cytoplasm, testifying to the intense activity of protein synthesis since the plasma cell is specialised in the synthesis of immunoglobulins (= antibodies). |
Cytokine synthesis
These cytokines are of crucial importance for T/B cell cooperation, which allows total and complete activation of these two cell types (see T cell activation / B cell activation).
Plasma cells: synthesis of antibodies (or immunoglobulins)
At the end of its activation, B cell becomes a plasma cell specialised in the synthesis of antibodies (immunoglobulins). These antibodies are of major importance for:
- Complement activation
- ADCC (Antibody Depedant Cell Cytotoxicity)
- Opsonization
What needs to be remembered
B cells are effector cells of adaptive immunity, particularly humoral, because plasma cells are the only cells capable of producing antibodies. These antibodies are of major importance in the context of transplantation, which is why B cells are the target of immunosuppressants. There are also techniques to eliminate or render non-functional antibodies.
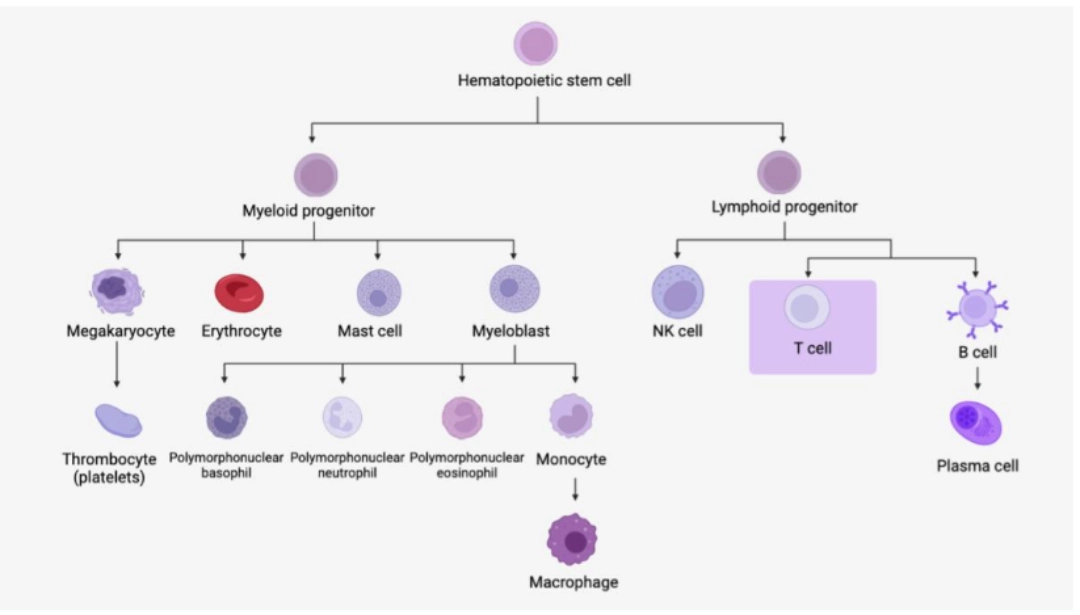
HAEMATOPOIESIS / CYTOLOGYCytology characteristics and the main principles of T cell ontogeny are described here. If the morphology of CD4+ and CD8+ T lymphocytes does not allow them to be distinguished, flow cytometry makes it possible to identify clusters of differentiation (CDs), and in particular CD4 and CD8 which make it possible to divide T cells into these two very different families on a functional level. |
 |
FUNCTIONS
Common features of CD4+ and CD8+ T Cells
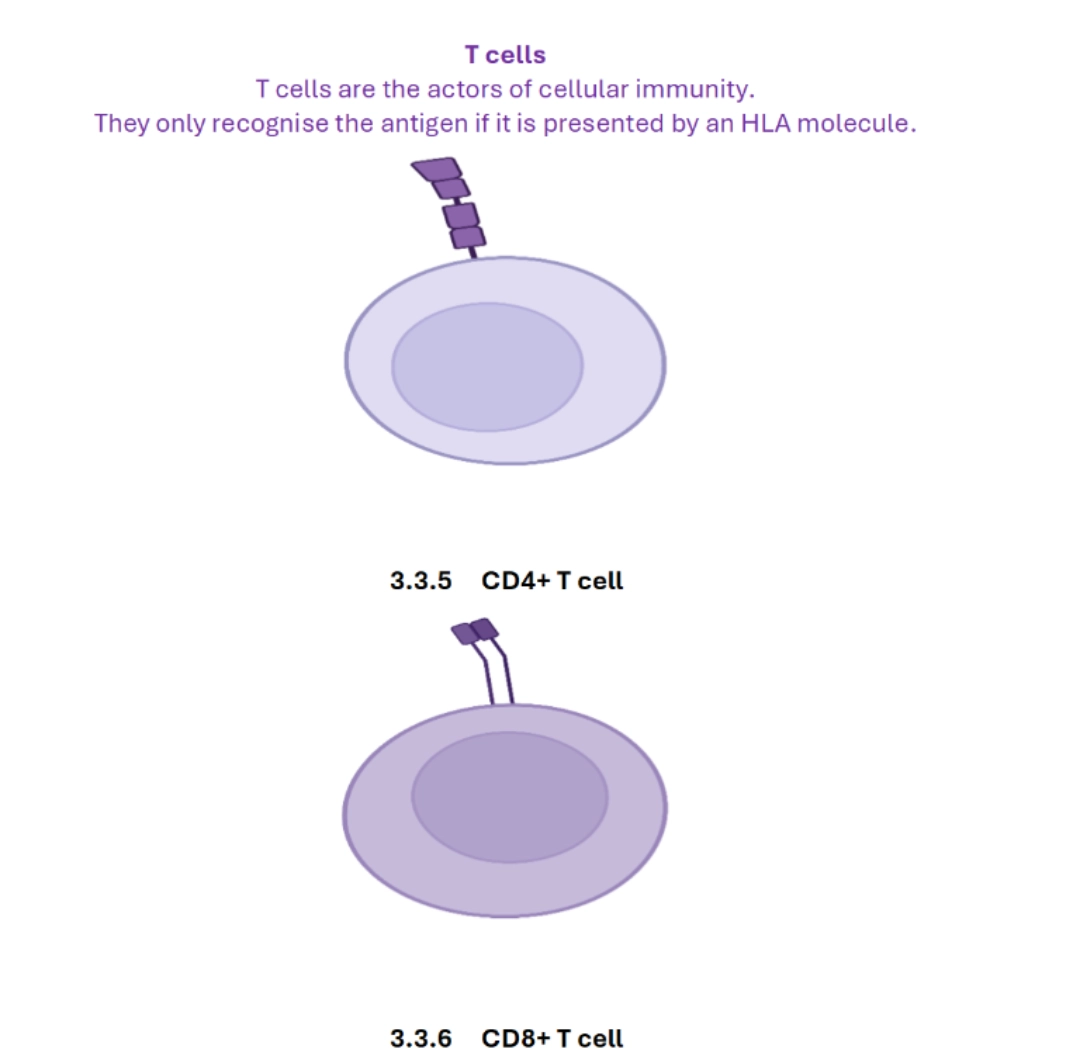
1. Antigen recognition
- The antigen is recognised if and only if it is embedded in an HLA molecule. It should be noted that only antigens of a protein nature and cleaved into very small fragments (peptides) can be embedded in HLA molecules to then be presented to lymphocytes.
2. Maturation
- T cells undergo a maturation process through which they acquire their TCR (see T cell ontogeny).
3. Activation
- CD8+ and CD4+ T cells are activated in the same way by the dendritic cell (see T cell activation).
Differences between CD4+ and CD8+ T cells
| CD8+ T cells | CD4+ T cells | |
|---|---|---|
| Name | Cytotoxic T cells (CTL) | T helper (Th) cells = Helper T cells |
| Co-receptor | CD8 | CD4 |
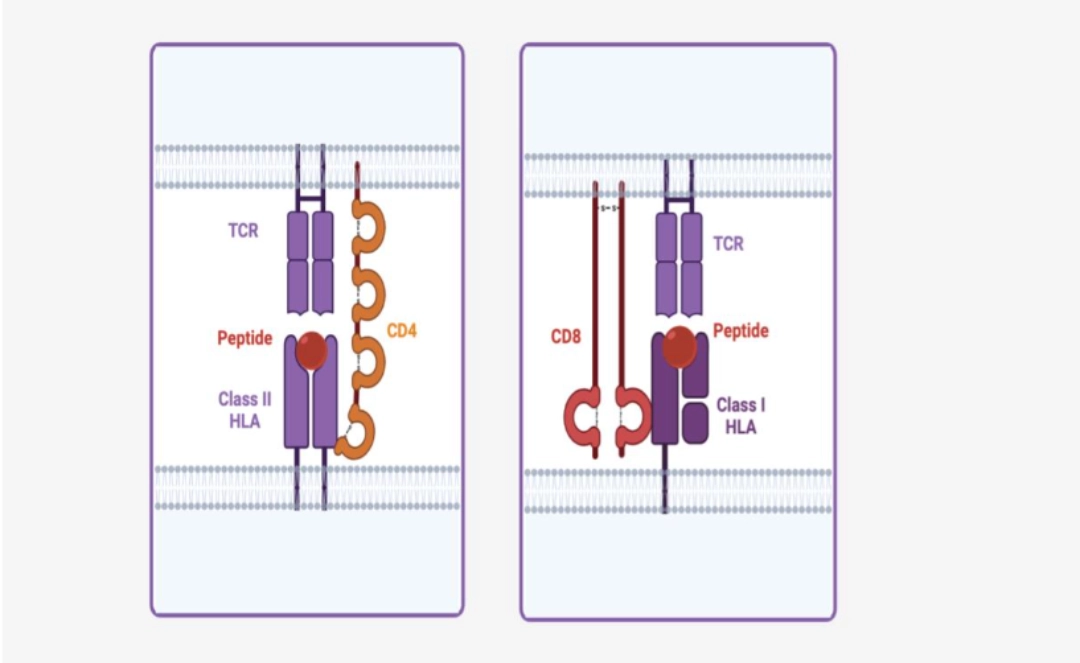
| Interaction | Class I HLA | Class II HLA |
| Peptides recognised | Endogenous (and exogenous by cross-presentation carried out by the dendritic cells) | Exogenous |
| Functions | Cytotoxicity | Cell cooperation with CD8+ T cells and B cells |
| Effector mechanisms | Perforin/granzyme, Fas – Fas-Ligand, TRAIL | Cytokine synthesis (Th1, Th2, Th17 profiles) |
What needs to be remembered
T cells include CD4+ and CD8+ T cells, which are two very heterogeneous populations in terms of functions. Their involvement in allorecognition mechanisms on the one hand, and the cytotoxic functions of CD8+ T cells on the other hand, are essential in organ transplantation, which is why they constitute the preferred target of current immunosuppressants.
MOLECULAR STRUCTURE
Functional classification
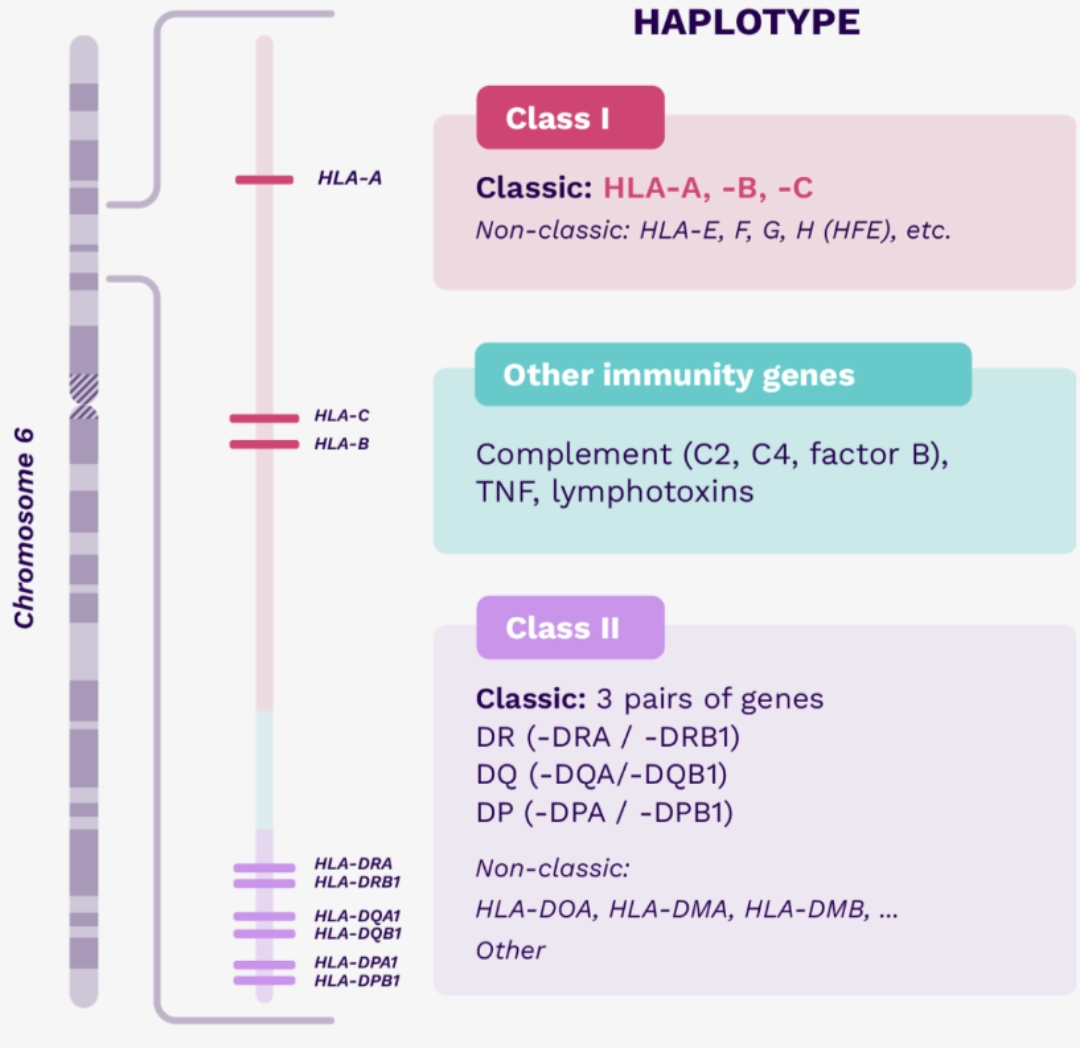
We distinguish between classical HLA molecules whose main function is the presentation of peptides to T cells, and non-classical HLA molecules , which have other functions (in the preparation of peptides, presentation of antigens of different types, to different cells, etc.).
| Class I HLA | Class II HLA |
|---|---|
|
Classical (HLA Ia) HLA-A HLA-B HLA-C |
Classical (HLA IIa) HLA-DR HLA-DQ HLA-DP |
|
Non-Classical (HLA Ib) HLA-E HLA-F HLA-G MICA/B |
Non-Classical (HLA IIb) HLA-DM HLA-DO |
Non-classical HLA molecules are major actors of the immune system, such as HLA IIb HLA-DM and HLA-DO molecules that play a role in the dismantling of the [Class II CLiP-HLA] complex, thus allowing the loading of antigenic peptides. Non-classical HLA molecules are not developed here.
CHARACTERISTICS OF THE CLASSICAL HLA SYSTEM
Antigen presentationIt is the main function of Class I and Class II HLA molecules (see antigen presentation).
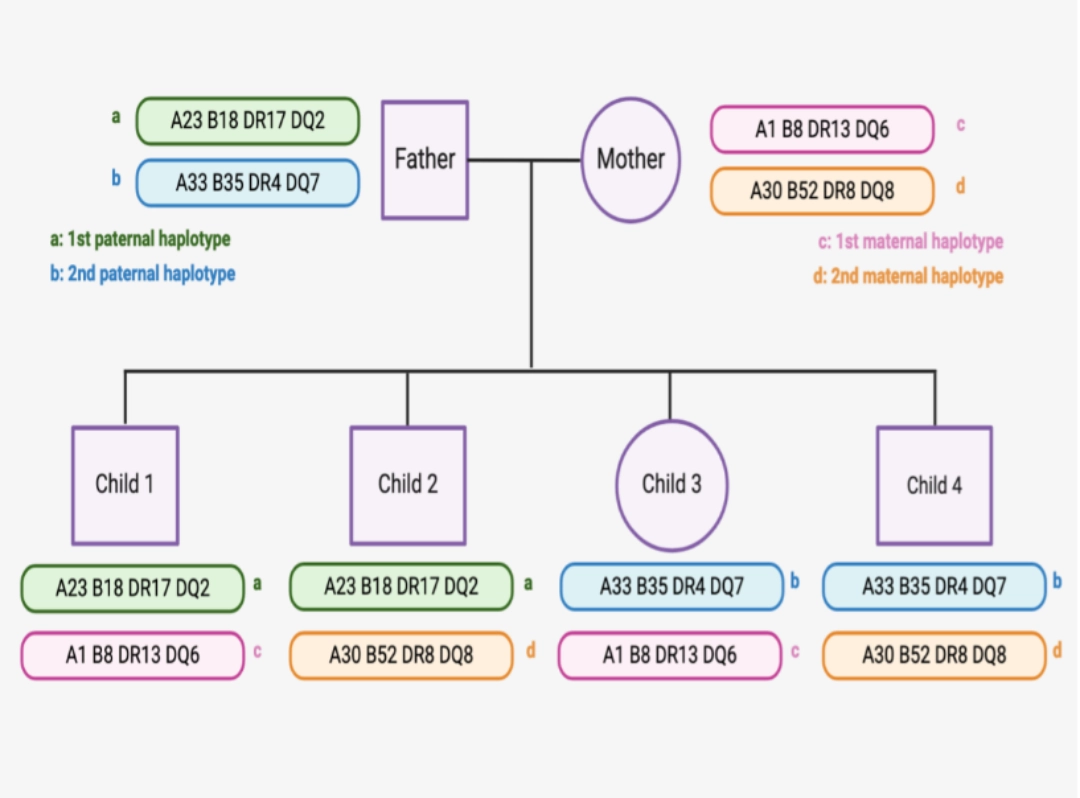
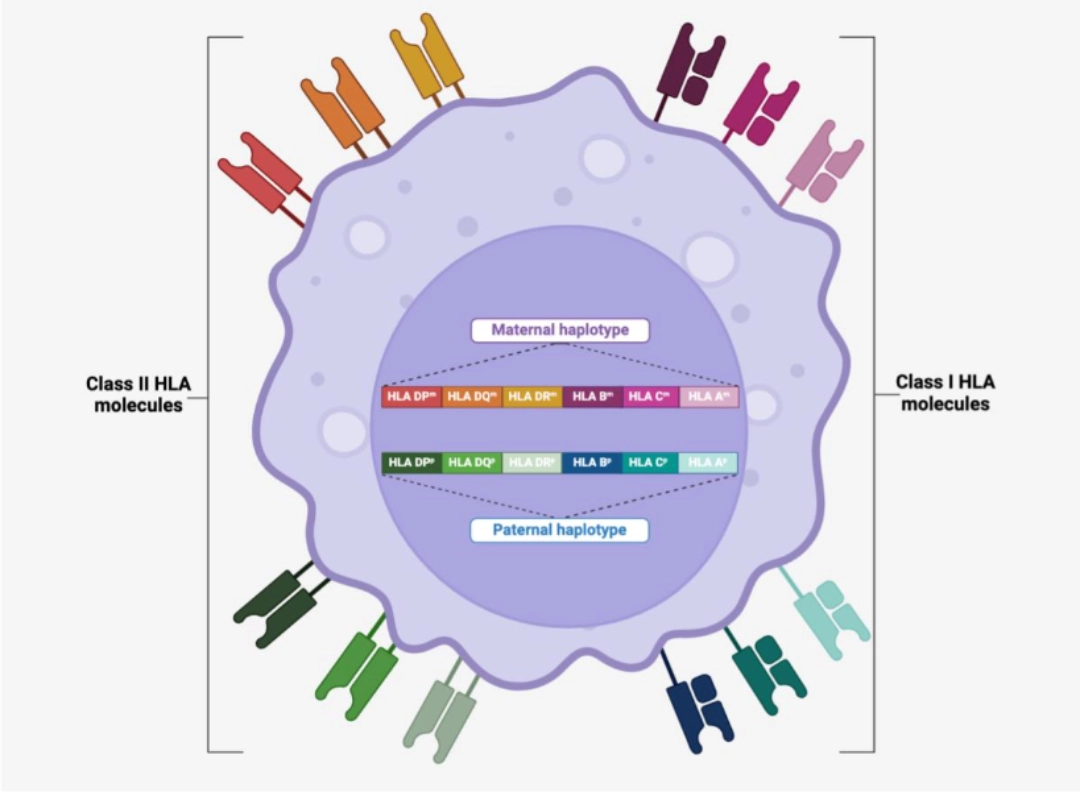
Haplotype and block transmission |
 |
Polymorphism
The genes encoding classical Class I and Class II HLA molecules are the most polymorphic in the human species. Alleles encode proteins that differ by one or more amino acids compared to other alleles, but the number of different proteins is fewer than alleles due to redundancy in the genetic code
Codominance
In addition to very high protein diversity and transmission by
haplotype, phenotypic polymorphism is major, because most individuals are heterozygous, and express on the cell surface the molecules of the haplotype inherited from the father, and the haplotype inherited from the mother.
Polymorphism of HLA genes
The polymorphism of HLA genes studied in clinical practice is deliberately reported in approximate numbers because the number of known alleles and proteins is constantly increasing, particularly since the massive sequencing of HLA genes.
See http://hla.alleles.org/nomenclature/stats.html for updated figures.
| Class I HLA | |||||||||
| Gene | A | B | C | ||||||
| Alleles | ≈7,800 | ≈9,200 | ≈7,800 | ||||||
| Proteins | ≈4,500 | ≈5,500 | ≈4,400 | ||||||
| Class II HLA | |||||||||
| Gene | DRA | DRB1 | DRB3 | DRB4 | DRB5 | DQA1 | DQB1 | DPA1 | DPB1 |
| Alleles | ≈45 | ≈3,500 | ≈460 | ≈230 | ≈190 | ≈580 | ≈2,400 | ≈550 | ≈2,300 |
| Proteins | 5 | ≈2,200 | ≈340 | ≈150 | ≈140 | ≈280 | ≈1,500 | ≈260 | ≈1,300 |
Expression of HLA molecules
- The expression of classical HLA genes depends on the cell type, and is influenced by the cellular environment, and in particular pro-inflammatory cytokines.
- Class I HLA molecules are expressed by all nucleated cells as well as platelets. A higher density is observed on lymphocytes, monocytes/macrophages, dendritic cells.
- In the basal state, the expression of Class II HLA molecules is low and limited to professional antigen-presenting cells : dendritic cells, macrophage, B cells. When these cells are activated, the density of expression of Class II HLA molecules increases. Class II HLA molecules are also found on thymic epithelial cells.
In an inflammatory context (as is the case in organ transplantation!), it should be noted that epithelial and endothelial cells can express Class II HLA molecules. On the other hand, the activation of quiescent T cells can induce the expression of Class II HLA molecules.
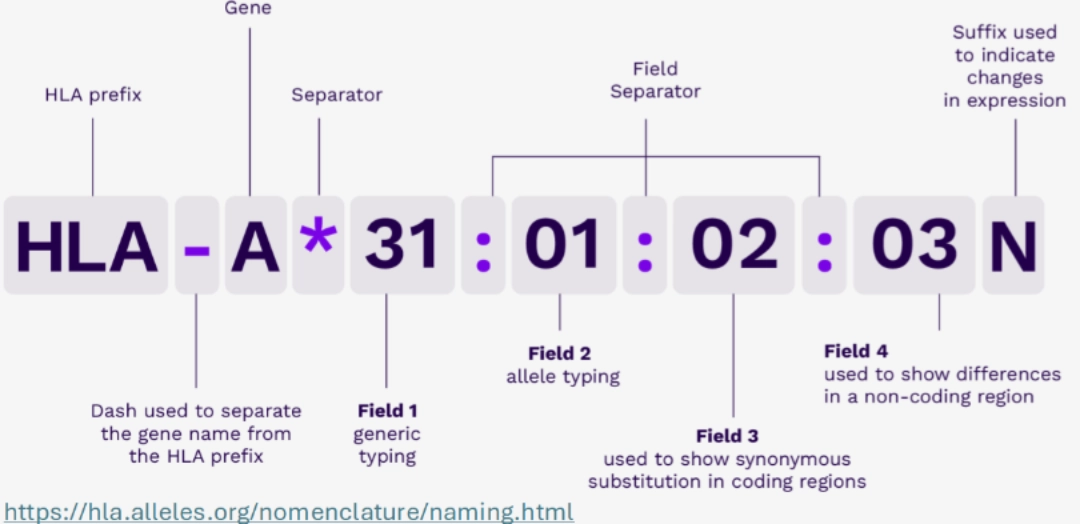
HLA NOMENCLATUR
- HLA: HLA region
- HLA-A: HLA-A gene
- HLA-A*31: group of alleles that encode HLA molecules corresponding to the A31 antigen
- HLA-A*31:01: a particular HLA-A*31 allele
- HLA-A*31:01:02: an allele that differs from the HLA-A*31:01:01 allele by a synonymous variation.
- HLA-A*31:01:02:03: an allele that differs from the HLA-A*31:01:02:01 allele by a mutation outside the exons.
Sometimes, the name ends with a suffix, and in particular the letters:
What needs to be remembered
Overall, the classical HLA system is involved in the discrimination of “self” and “non-self” through peptide presentation. Allelic and phenotypic polymorphism leads to the uniqueness of the adaptive immune response, involved both in protection against infectious agents, but also in anti-tumour monitoring, solid organ and haematopoietic stem cell transplantation, and susceptibility to certain autoimmune diseases.
|
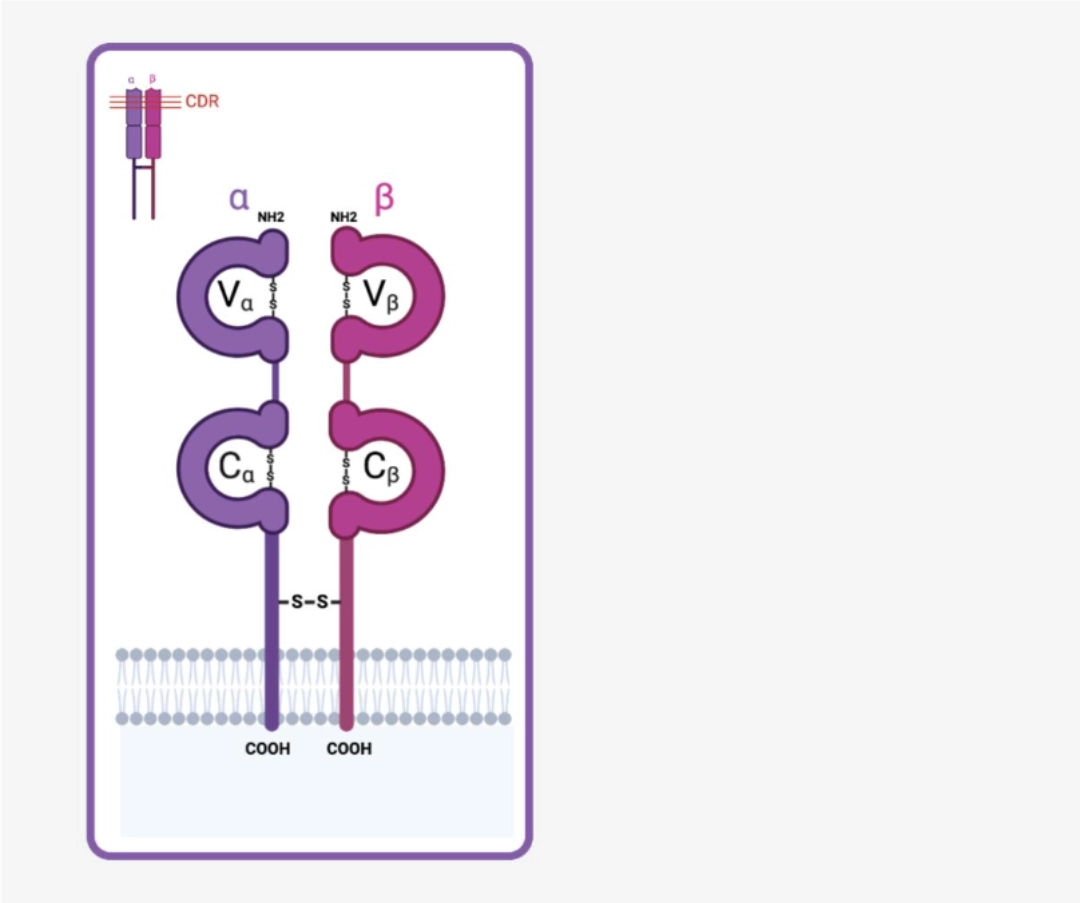
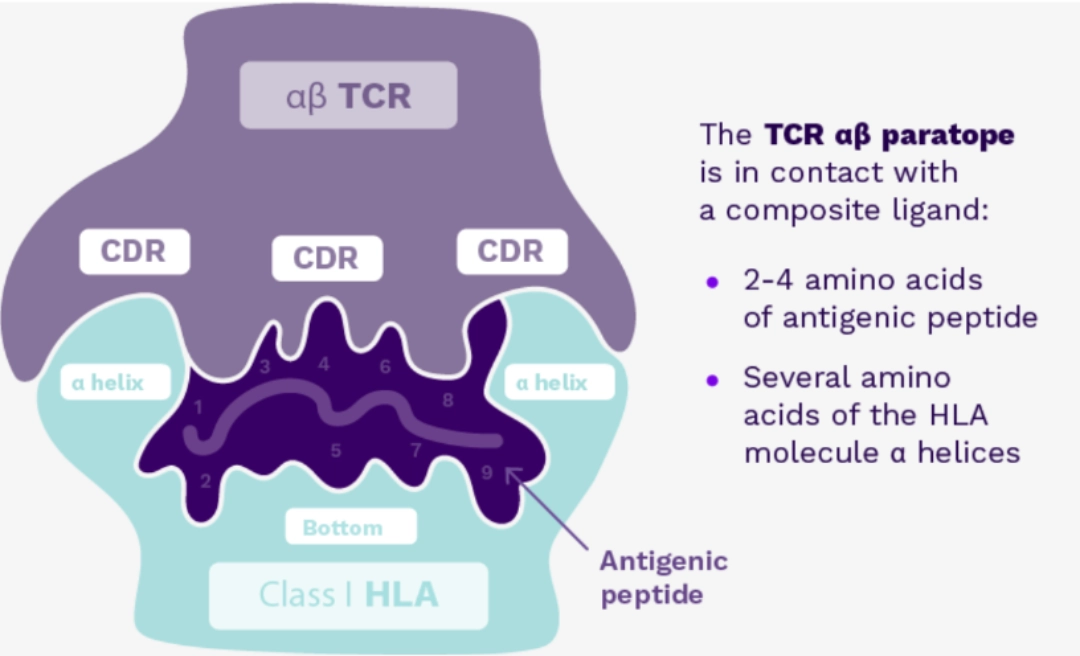
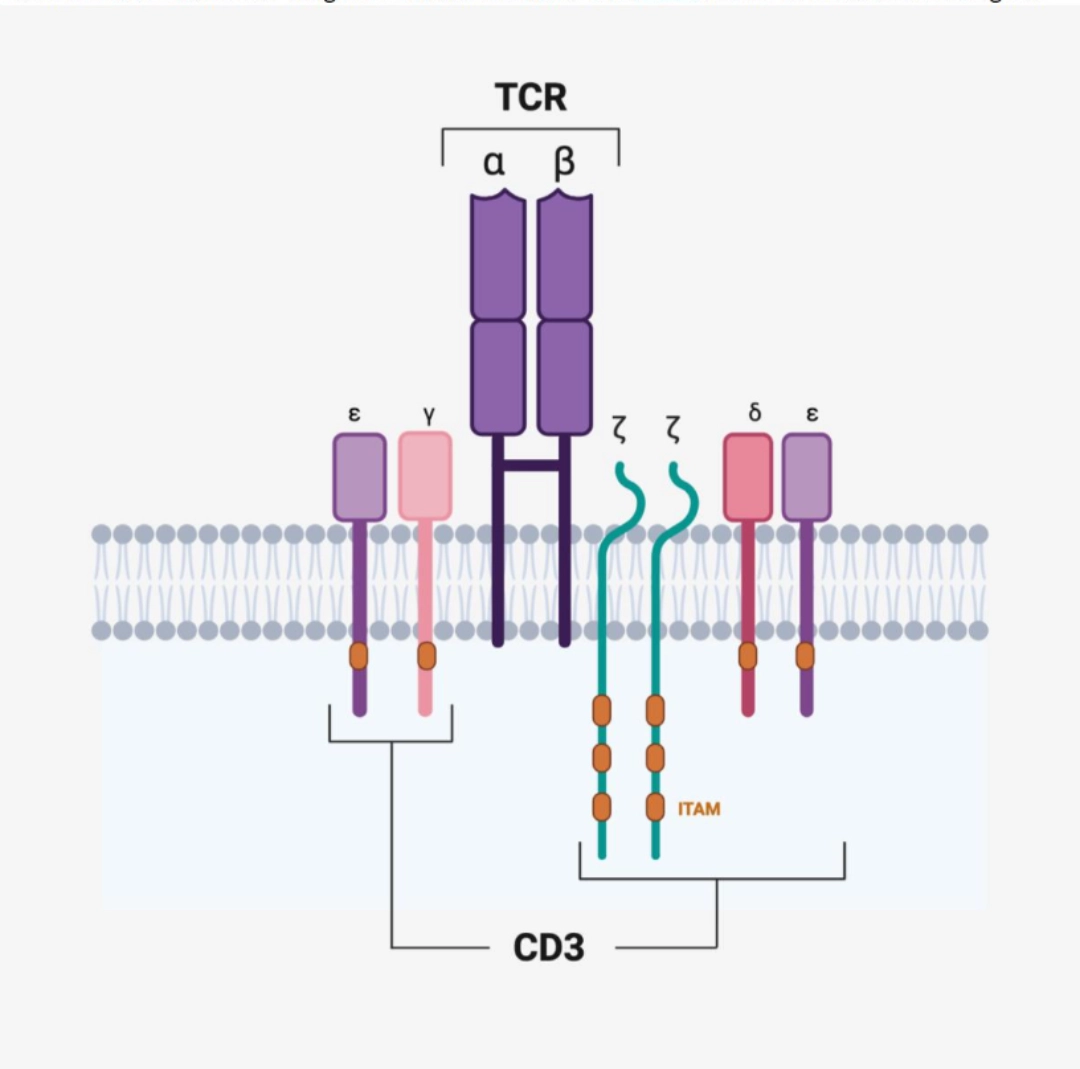
The TCR (T cell Receptor) is the specific antigen receptor for both the CD8+ T cell and the CD4+ T cell. It is the result of the somatic recombination of genes V and J of the α chain, and V D and J of the β chain. The TCR recognises a peptide antigen presented by a Class I or Class II HLA molecule (see antigen presentation), while signal transduction is ensured by the co-receptors. |
 |
To go further:
There are γδ TCRs expressed by γδ T cells, representing approximately 10% of the T cell contingent, and present mainly in the mucous membranes. The γδ TCRs are notably much less variant than the αβ TCRs. Some of them can recognize antigens embedded in Class I or Class II HLA molecules; however, most recognise:
- lipids or glycolipids embedded in non-classical HLA molecules
- either heat shock proteins from self
- or proteins of microbial origin
To simplify the matter, only the αβ TCRs will be described below.
V(D)J TCR RECOMBINATION
Each T cell synthesises a single type of TCR in very multiple copies. In other words, the T cell must only accept the rearrangement of variable region genes of a single chromosome 7 (β chain) and a single chromosome 14 (α chain), in accordance with allelic exclusion. The rearrangement of the β chain occurs first. If it is complete, then the rearrangement of the α chain can begin. The T cell precursor can only survive if the rearrangement of the α and β chains is productive, i.e. leading to the synthesis of a complete αβ TCR. |
J-TCR-Recombination.png/jcr:content/V(D)J%20TCR%20Recombination.png) |
Number of functional gene segments involved in V(D)J TCR recombination
| Chromosome | V | D | J | |
|---|---|---|---|---|
| α chain | 14 | ≈40 | 0 | ≈50 |
| β chain | 7 | ≈40 | 2 | ≈15 |
See adaptive immunity receptors for more details
ENGAGEMENT AND DOWNSTREAM PATHWAYS
Once the TCR engages in the [antigen-HLA] interaction, signal transduction occurs which leads to the activation of downstream molecular pathways. (see T cell activation).
What needs to be remembered
The TCR is the immunoreceptor carried by T cells, and each of them carries on its surface the same TCR in very numerous copies. The TCR is the product of somatic V(D)J recombination, which makes it possible to generate an infinite number of receptors to respond to the infinite number of antigens present in our environment. Since the TCR generation process is completely random, T cells undergo a positive and negative selection step to avoid the generation of self-reactive clones.
- Class I HLA molecules, as well as all nucleated cells in the body.
- Class II HLA molecules: little present in the basal state, they are nevertheless particularly quick to be expressed during cellular stress or an inflammatory situation.
Cellular stress or inflammatory situation: as in organ transplantation!
THE ABO SYSTEM IS A TISSUE SYSTEM
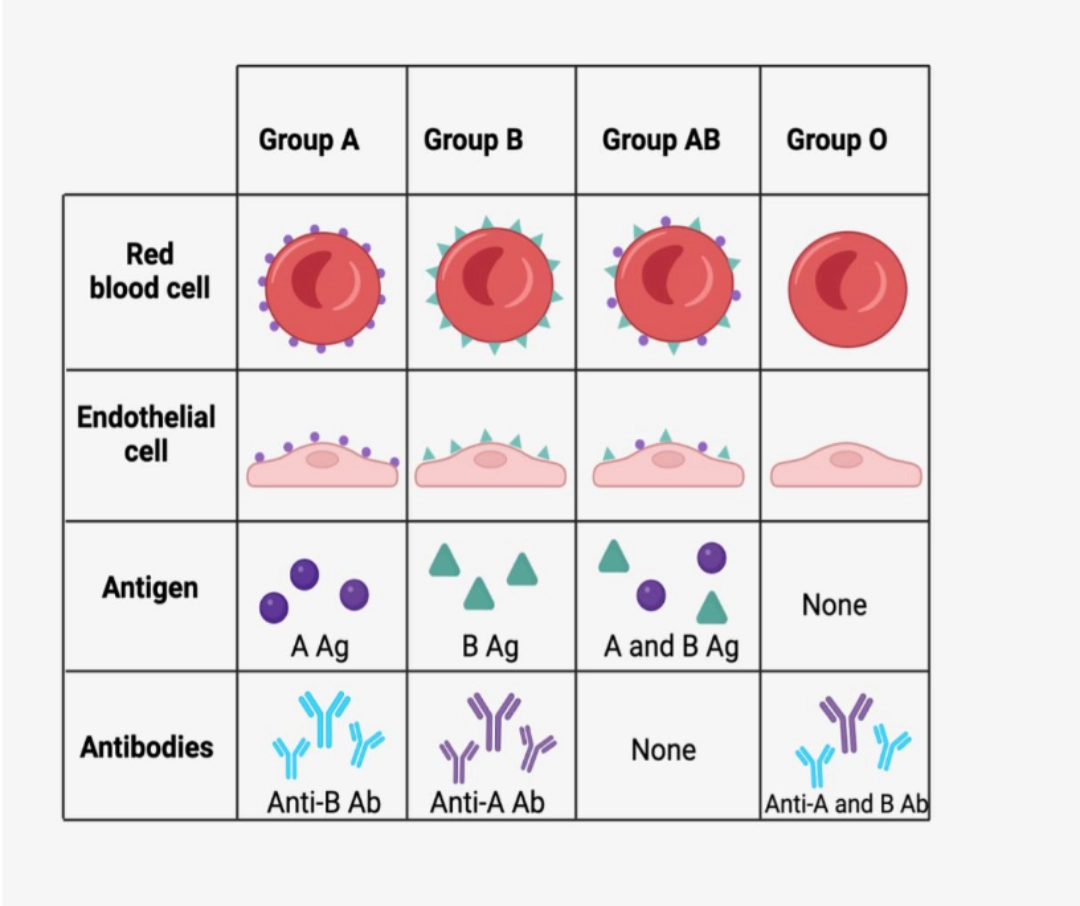
ABO antigens are not carried only by red blood cells, but by many tissues including endothelial cells, to the point that the ABO system can be considered a histocompatibility system!
To go further:
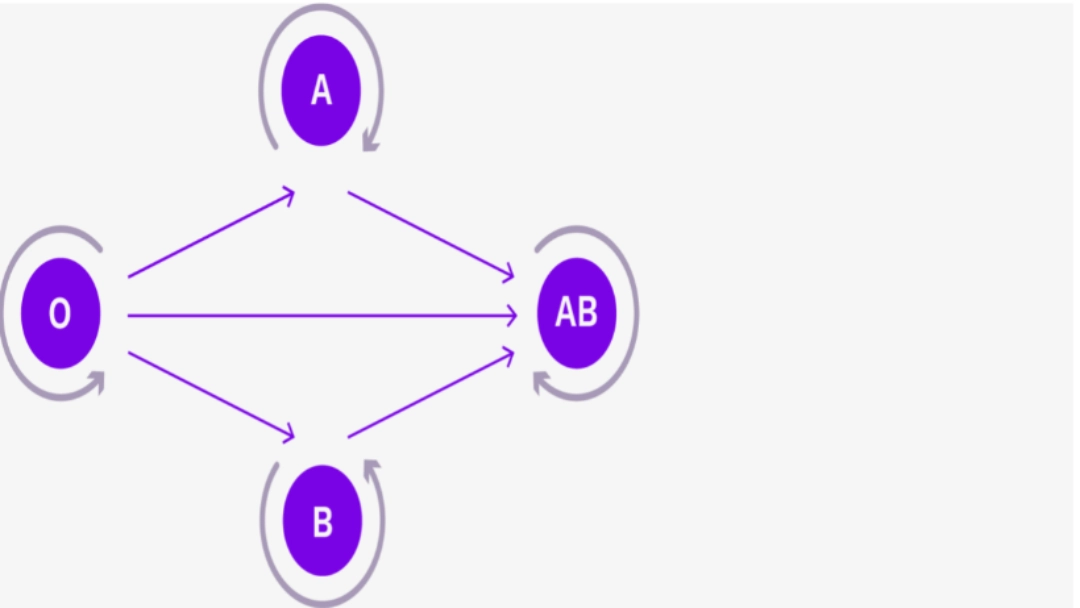
Group O individuals are universal donors in the absence of antigen present on the surface of red blood cells and endothelial cells. On the other hand, the presence of anti-A and anti-B antibodies in their serum makes group O recipients only compatible with individuals of the same blood group.
Conversely, group AB individuals are universal recipients in the absence of anti-A and anti-B antibodies present in their serum. They can only give to AB recipients, all other groups having anti-A and/or anti-B antibodies
Compatibility in the ABO system
| Recipient | A | B | AB | O |
|---|---|---|---|---|
| Donar | ||||
| A | isogroup | incompatible | compatible | incompatible |
| B | incompatible | isogroup | compatible | incompatible |
| AB | incompatible | incompatible | isogroup | incompatible |
| O | compatible | compatible | compatible | isogroup |
If we transplant a recipient with antibodies directed against an ABO antigen present in the donor (example of a recipient with blood group O and a donor with blood group other than O), it is almost certain that hyperacute rejection occurs due to the antibodies preformed in the recipient, which will directly agglutinate against the antigens on the surface of the endothelial cells, immediately leading to complement activation, and then an entire inflammatory cascade leading to graft necrosis.
What needs to be remembered
Compatibility in the ABO system is a major criterion for the success of the transplant, just like HLA compatibility. On the other hand, rhesus compatibility is not a criterion for allocation of grafts, given that rhesus antigens are not carried by the tissues.
Cytokines are the soluble molecules emitted by the different actors of immunity in order to ensure temporal-spatial coordination. Indeed, all the elements contributing to the immune response are very heterogeneous, both in space (the different actors are in different places in the body at a given time), and in time (the different actors are at different stages of maturation at a given time).
Given the finesse and adaptability of the immune response, the range of information exchanged must necessarily be very broad. Thus, cytokine diversity is very important to meet this need.
The different names of cytokines
Cytokines refer to all molecules allowing communication between immune cells. However, we can find other names for these molecules.
- Interleukins (abbreviated IL): “between leukocytes”. However, it tends to be abandoned because this classification is outdated (example of TNF which has all the properties of an interleukin but not the name).
- Chemokines: subcategory of cytokines having in particular a chemoattractant power, that is to say inducing cell mobility.
MOLECULAR DESCRIPTION
Cytokines are small proteins with a molecular mass around 30kDa. Most are glycosylated which contributes to stability but is not necessary for cytokine activity.
COMMON PROPERTIES OF CYTOKINES
- Pleiotropy: a cytokine may induce different effects.
- Redundancy: two different cytokines may have the same effect.
- Synergy: the combined effect of two cytokines is not necessarily the addition of the effect of each cytokine, but can induce a tenfold or different effect.
- Antagonism: the effects of one cytokine can inhibit those of another cytokine.
- Induction of cascades: the action of a cytokine on a target cell can induce the secretion of other cytokines with different effects.
DIVERSITY OF CYTOKINES
Diversity of functions
Cytokines regulate the intensity and duration of the immune response because they are responsible for the mechanisms of activation, proliferation, and differentiation of target cells. They have countless functions, including:
the change in expression of membrane molecules (adhesion molecules, activation co-signals, HLA molecules).
the change in expression of membrane receptors (receptors for different cytokines, whose conformation changes modify their affinity for cytokines).
the change of transcriptional program.
the change in organisation of the cytoskeleton and organelles.
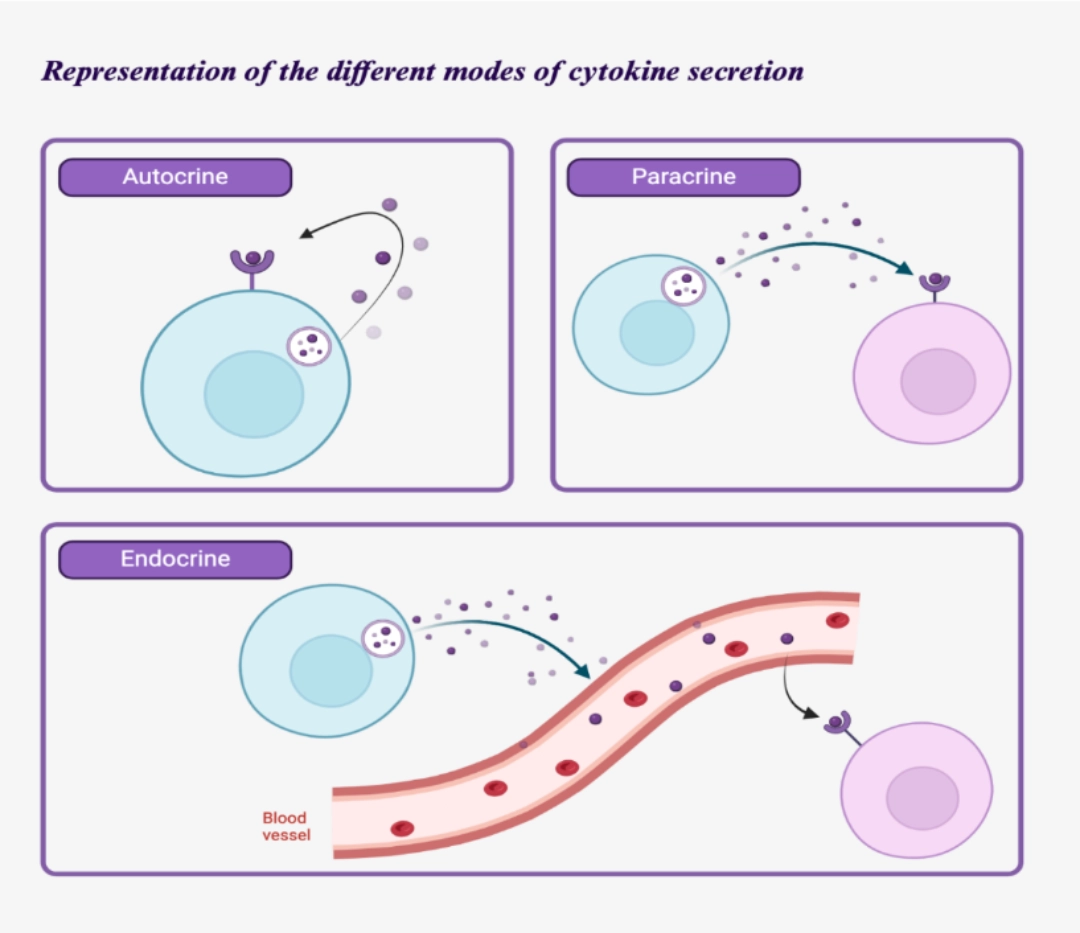
the change in cytokine secretion...
These secretion modes are not exclusive (e.g., IL-2 acts via these three secretion modes).
Immunity diversity
There are innate immunity cytokines, such as adaptive immunity cytokines, and cytokines involved in both innate and adaptive immunity (e.g., type 1 interferon).
Location diversity
The overwhelming majority of cytokines are soluble, although some cytokines can be membrane bound.
Classification of cytokines
Six families of cytokines are described:
- Interleukin-1 family: primarily involved in pro-inflammatory innate immunity, including increased capillary permeability, lymphocyte recruitment, and systemic effects of inflammation.
- Haematopoietin family: historically includes the cytokines effectively involved in hematopoiesis, whose tertiary configuration is based on a bundle of four antiparallelhelices. By extension, other cytokines with the same structure have been categorised in this family, although their functions are not essentially haematopoietic, but as diverse as the proliferation of lymphocytes, differentiation of B cells into plasma cells, etc.
- Interferon family: There are three major types of interferons, all of which increase the expression of Class I and II HLA molecules on the surface of cells.
- Type I interferons: interferon α and interferon β, secreted after recognition of viral patterns by the PRRs. Interferon α is secreted by activated macrophages and dendritic cells, while interferon β is secreted by fibroblasts.
- Type II interferons: γ interferon produced by NK cells and T cells, directs the immune response towards a cytotoxic response (increase in Th1 cells, activation of macrophages, differentiation of CD8+ T cells)
- Type III interferons: interferon λ, interferon delta, interleukin 10 (IL-10)
- Family of tumour necrosis factors: these are particular cytokines, because they are most often anchored in the cell membrane.
- Interleukin 17 family: recently discovered, this family brings together mainly pro-inflammatory cytokines at the interface of innate and adaptive immunity.
- Chemokine family: Tnes at the interface of innate and adaptive immunity. · Chemokine family: they include cytokines specialised in attracting different elements of the immune response. This attraction occurs according to a concentration gradient of chemokines, which attract cells possessing the dedicated receptor towards the source of synthesis. These chemokines can attach in particular to the walls of the vessels, and therefore guide the elements of immunity in the vessels.
CYTOKINE RECEPTORS
Each cytokine can bind to more than one receptor and a receptor can bind multiple cytokines. In total, around twenty receptors recognise around thirty cytokines. Most of the time, these receptors are transmembrane
The sensitivity of a cell to a cytokine stimulus is regulated by:
Signal intensity
the receptors sensing this signal (quantity of expression and affinity for the cytokine)
In general, there is a very strong affinity of the receptor for the cytokine which is secreted near the receptor (concept of immunological synapse).
Immunological synapses
Immunological synapses explain why systemic cytokine concentrations must becarefully interpreted , and the topography of cellularinteractions must be taken into account.
How can we explain that the same cytokine can have distinct effects on different cell types?
1. Cellular expression of cytokine receptors
The expression of cytokine receptors varies depending on the cell types, but also on their state of activation.
For example, a T cell after activation – that is to say after its encounter with the antigen – will overexpress the IL-2 receptor (proliferation signal) as well as the IL-4 receptor (differentiation signal). Thus the T cells which have encountered the antigen will continue their maturation/proliferation process, while the naive T cells (those which have not encountered the antigen) will remain inert until this encounter.
2. Co-signals
Signal integration and cellular response do not only depend on a cytokine but also:
- on other cytokines in the environment
- on receptors expressed at time T by the cell
- on the state of the cell and its environment
|
The primary lymphoid organs are the site of maturation of T and B cells, marked respectively by the acquisition of the TCR and BCR in the thymus and in the bone marrow (see T cell ontogeny and of B cell ontogeny). |
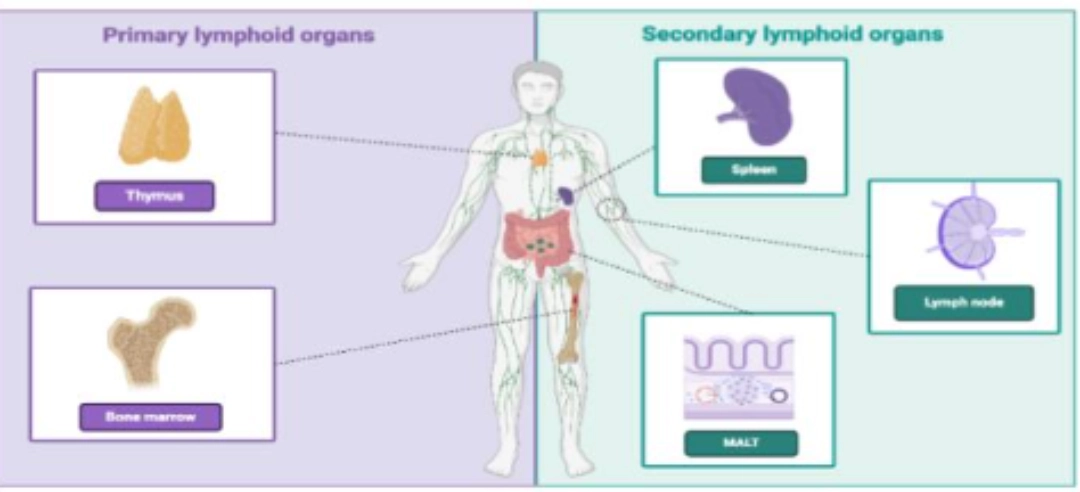 |
|
The secondary lymphoid organs are the preferred sites for the antigen /lymphocyte encounter. These organs are the sites of activation of T cells and B cells , and allow cellular cooperation of adaptive immunity. |
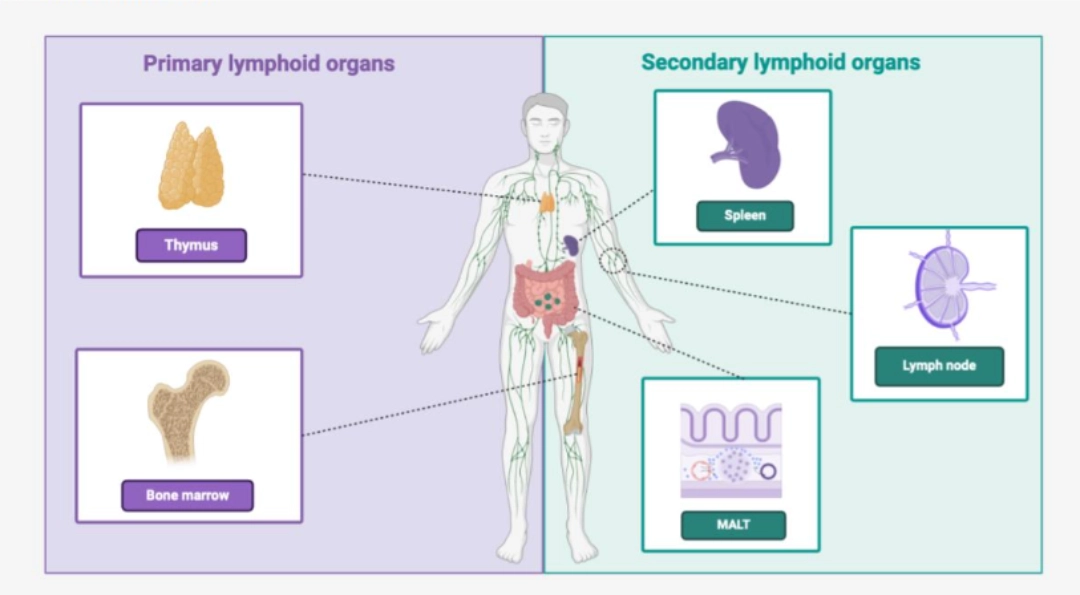 |
|
Note: MALT = Mucosa-Associated Lymphoid Tissue. Ces formations lymphoïdes ne seront pas développées ici. |
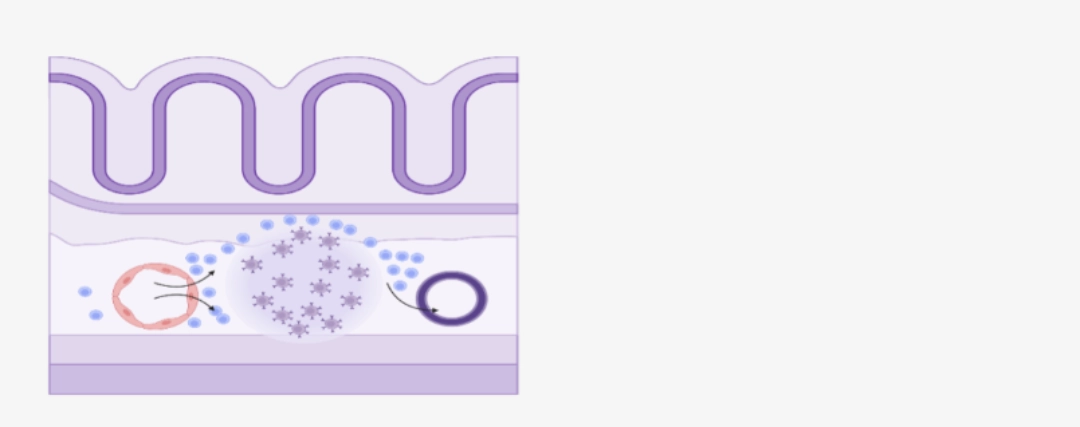 |
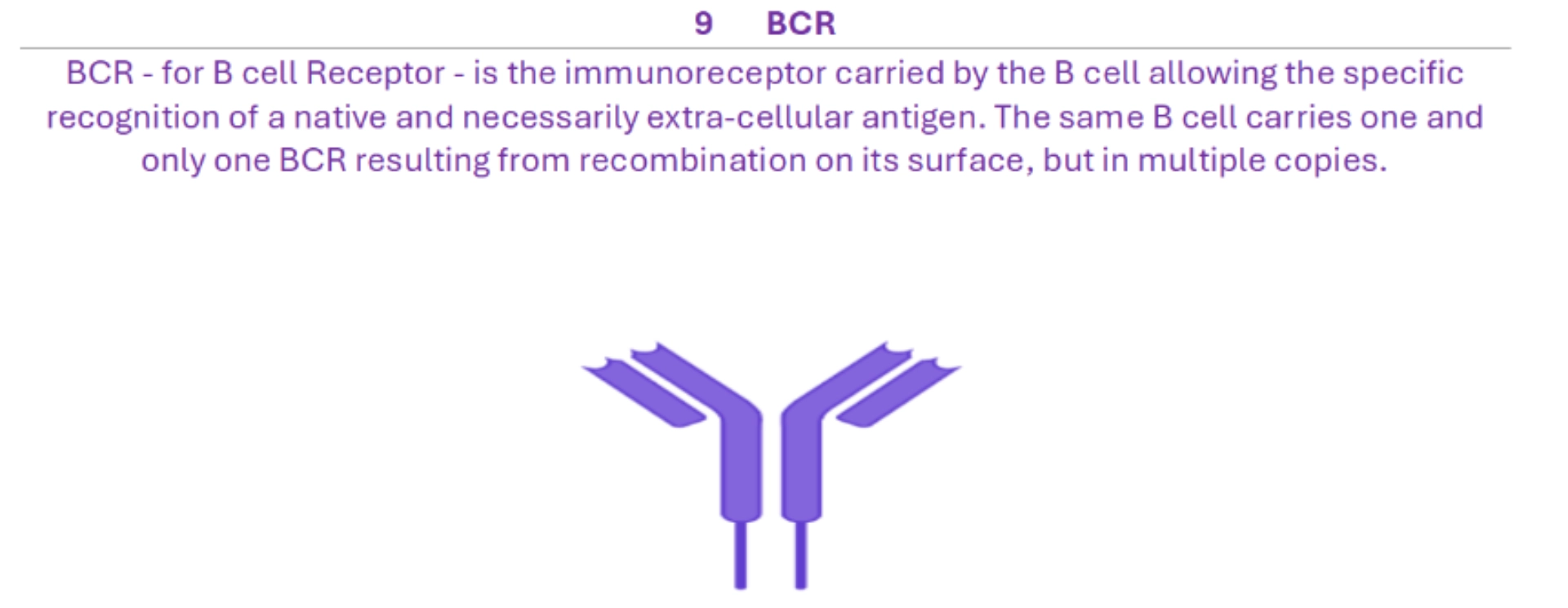
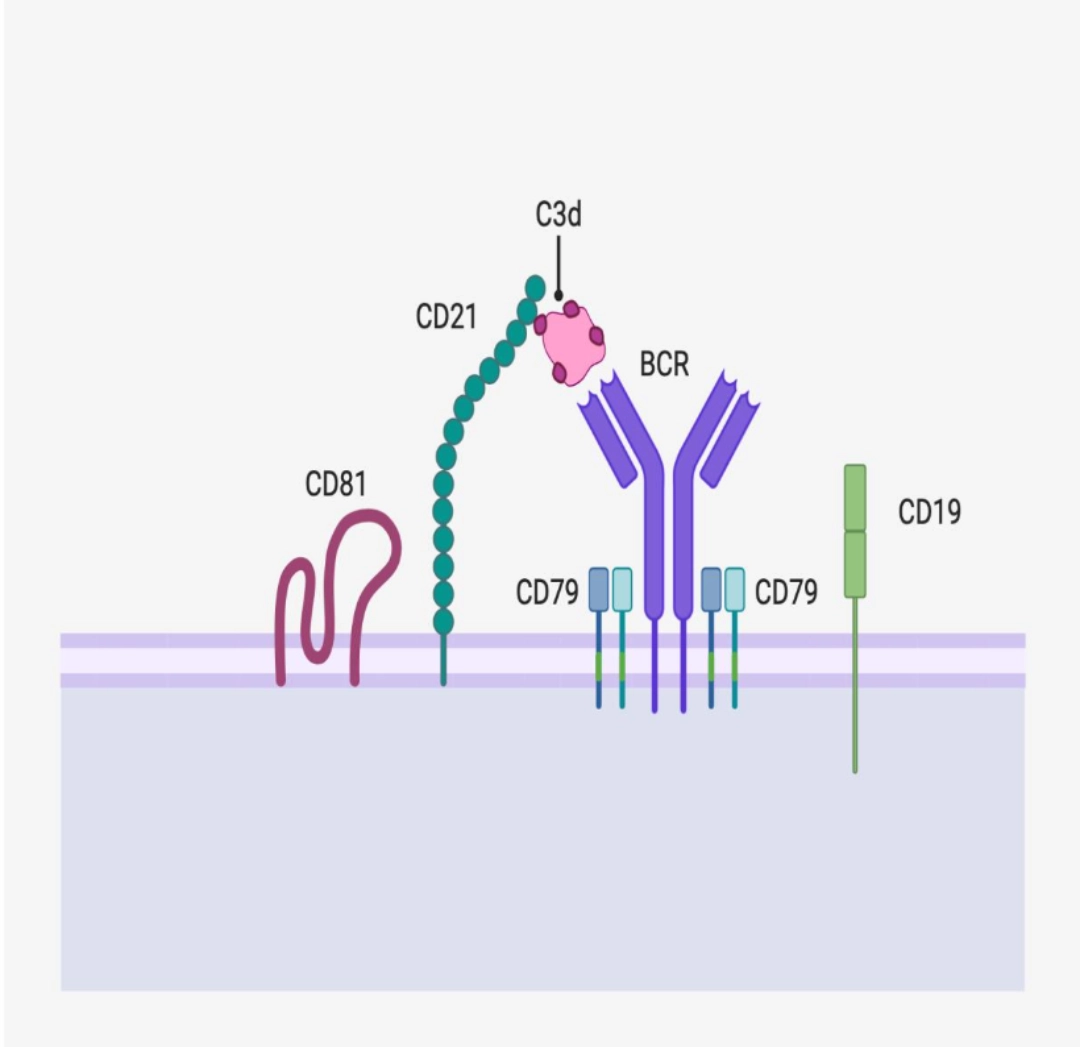
BCR (B Cell Receptor) is the specific antigen receptor of the B cell. It results from somatic recombination D-J then V-DJ of a heavy chain (µ then δ) and V-J of a light chain (κ or λ). BCR recognises a native antigen, necessarily extracellular, while signal transduction is ensured by a co-receptor. The plasma cell no longer has BCRs but secretes them in soluble form, also called immunoglobulins or antibodies. This is why BCR is also called “surface immunoglobulin”
TERTIARY STRUCTURE OF THE RECEPTORBCR is a short transmembrane receptor of the immunoglobulin superfamily. It is a heterotetramer formed of two light chains (κ or λ) and two heavy chains (µ or δ) identical in pairs. Each of these chains is the product of a V(D)J recombination, whose polymorphism is concentrated in the V variable regions (VL and HL respectively) to provide maximum diversity at the paratope level (Fab), while retaining the structure responsible for initiating signal transduction (Fc). Hypervariable regions (CDR Complementarity Determining Region) of the light chain variable region recognise the antigen (CDR1, CDR2, and CDR3). |
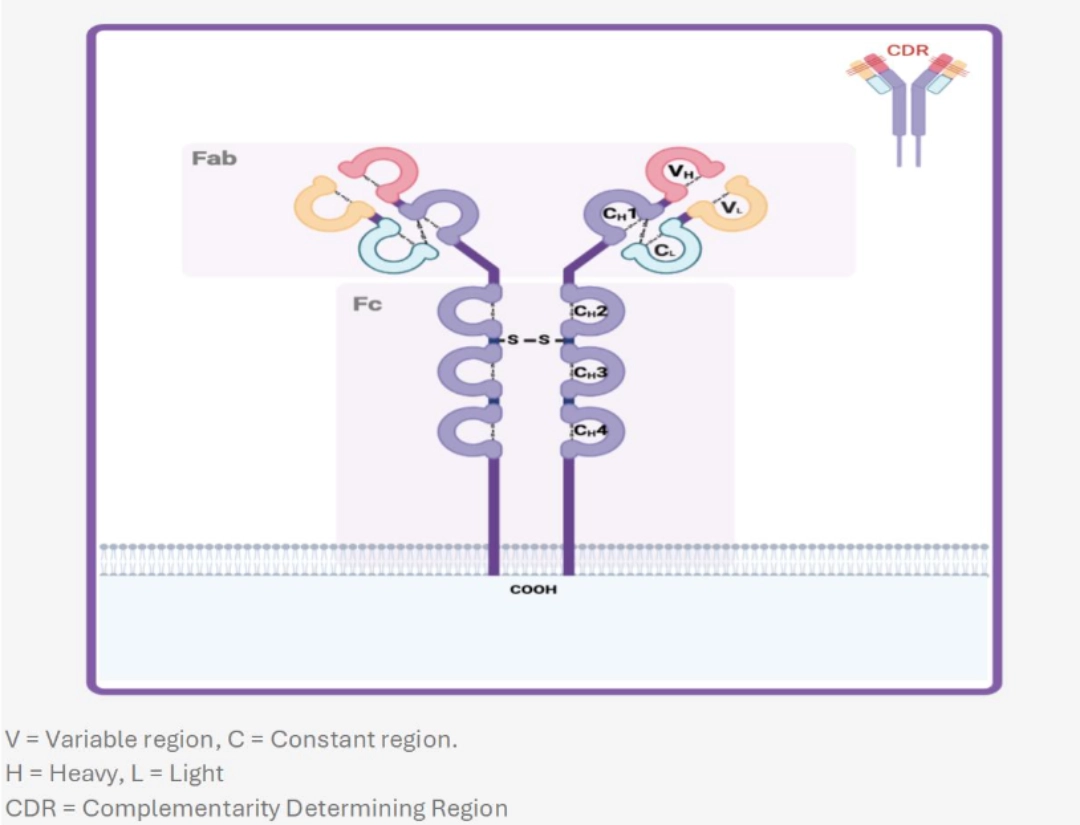 |
V(D)J RECOMBINATION OF THE BCR
Each B cell synthesises a single type of BCR in very multiple copies. In other words, the B cell must only accept the recombination of a single chromosome 14 (H heavy chain), in accordance with the principle of allelic exclusion and of one of the two chromosomes 2 (κ light chain) or chromosomes 22 (λ light chain) in accordance with the principle of isotype exclusion.
A BCR is ALWAYS made up of two heavy chains and two identical light chains in pairs. It is not possible to have a BCR with two different heavy chains or two different light chains.
The recombination of the heavy chain occurs first. If this is complete, then the recombination of the light chain can begin. The precursor of the B cell can only survive and differentiate into a B cell if the rearrangements of a heavy chain and a light chain lead to the synthesis of a BCR (1st and 2nd checks within the bone marrow) (see B cell ontogeny).
Number of functional gene segments involved in BCR V(D)J recombination
| Chromosome | V | D | J | |
|---|---|---|---|---|
| Light chain κ | 22 | ≈35 | 0 | 5 (but multiple alleles) |
| Light chain λ | 2 | ≈35 | 0 | 5 |
| Heavy chain | 14 | ≈35 | ≈25 | 6 |
CO-RECEPTORSCD79 ensures signal transductionCD79 is an Igα/Igβ heterodimer whose long intra-cytoplasmic part (unlike the BCR which only has 3 intracellular amino acids) allows signal transduction. |
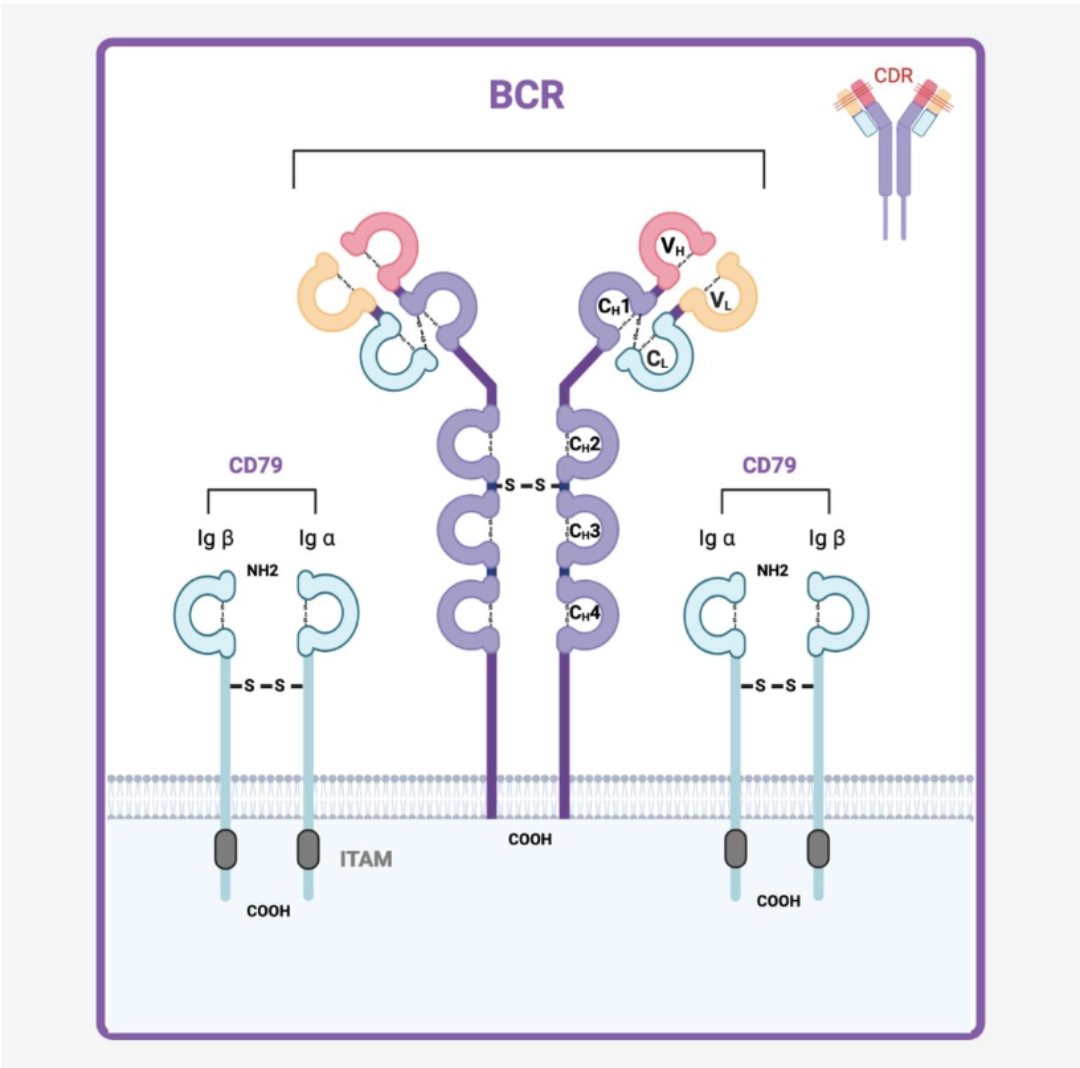 |
ENGAGEMENT AND DOWNSTREAM PATHWAYS
Once the BCR engages in the interaction with the native antigen, signal transduction takes place which leads to the activation of downstream molecular pathways (see B cell activation).
What needs to be remembered
The BCR is the immunoreceptor carried by B cells, and each of them carries on its surface the same BCR in very numerous copies. The BCR is the product of somatic V(D)J recombination, which makes it possible to generate an infinite number of receptors to respond to the infinite number of antigens present in our environment. After being activated, the B cell can transform into a plasma cell: it then no longer expresses BCR on its surface but secretes them, these are then immunoglobulins, also called antibodies.
Antigens can be classified:
According to their nature
Antigens can be protein, polysaccharide, lipid, or a mix of several types (for example a glycosylated protein). In practice, it is important to make the dichotomy between peptide antigens and others. This distinction is important because TCRs (on the surface of T cells) can only recognise protein antigens after priming – i.e., cleavage into small peptides of 9 amino acids for Class I, or 15 to 21 amino acids for Class II – while membrane immunoglobulins (BCRs) or soluble immunoglobulins (antibodies) can recognise antigens of all natures.
According to their persistence after denaturation
The molecules of the organism adopt a tertiary structure, that is to say that the peptide chain is organized in space to form a 3D structure defining, for example, domains of activity (for example: folding to form a catalytic site).
Under certain conditions (variation in temperature, acidity, oxidative stress, etc.), this conformation is broken and the peptide chain loses its 3D conformation and becomes linearised again.
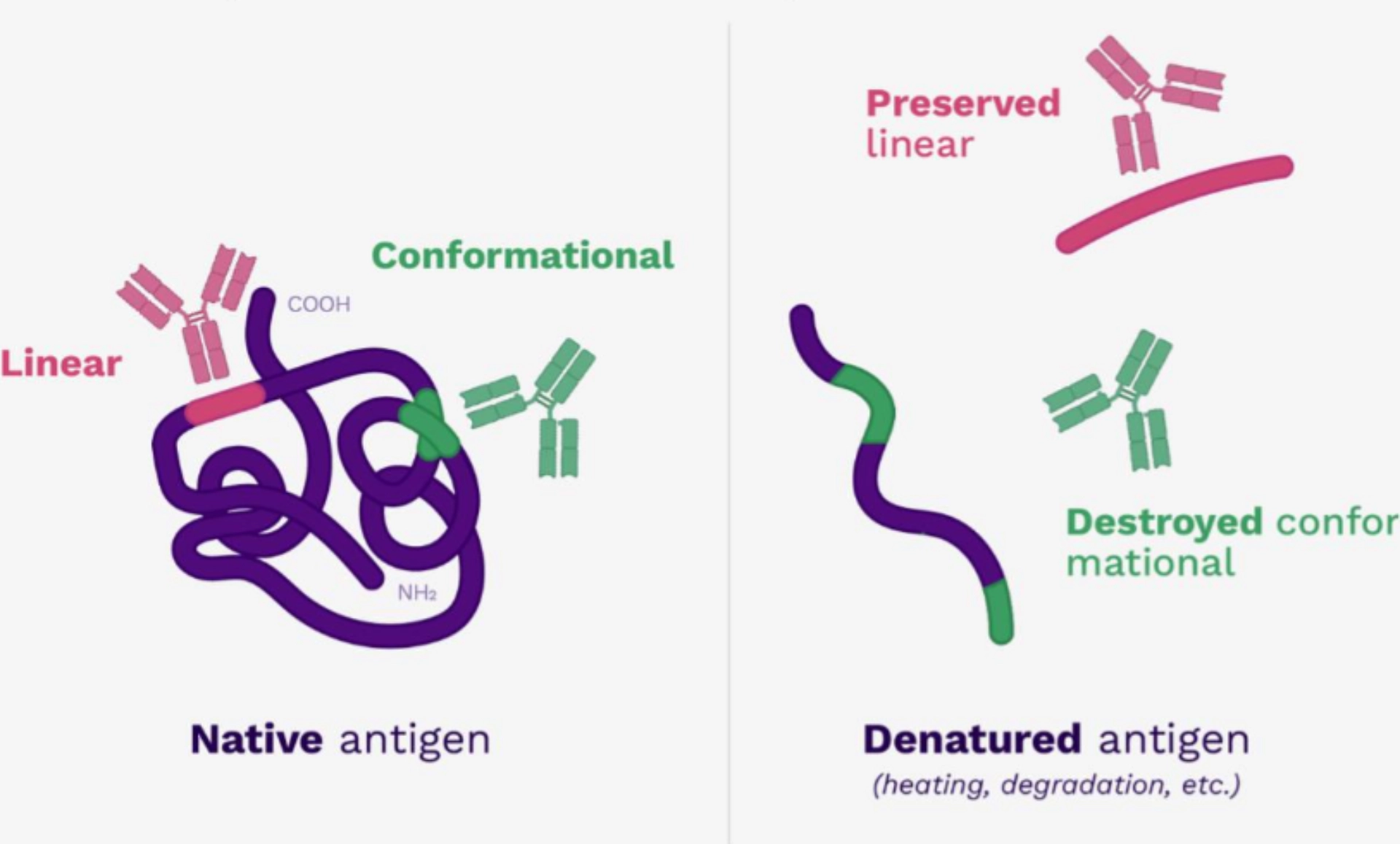
We therefore distinguish between:
- conformational antigens: they are composed of motifs that are not contiguous in the molecular sequence but close in the 3D structure.
- linear antigens: they are composed of contiguous motifs in the molecular sequence.
When the 3D structure is broken, the conformational antigen is lost but the linear antigen persists.
Since the antigens recognised by the TCR are processed, TCRs only recognise linear antigens (not conformational antigens).
BCRs can recognise conformational and linear antigens.
According to their source
- Microbial antigens: coming from microorganisms (bacteria, viruses, fungi, etc.).
- Allergens: substances in the environment that cause allergies (hypersensitivities).
- Alloantigens: coming from organisms of the same species but characteristics of subgroups of individuals within a given species (HLA molecules, ABO system, etc.).
- Xenoantigens: coming from organisms of different species (specific glycosylation of horse immunoglobulins for example, etc.).
- Autoantigens: Substances in self (individual) that become antigens, as in autoimmune diseases.
We speak of exogenous and endogenous antigen to the antigen-presenting cell:
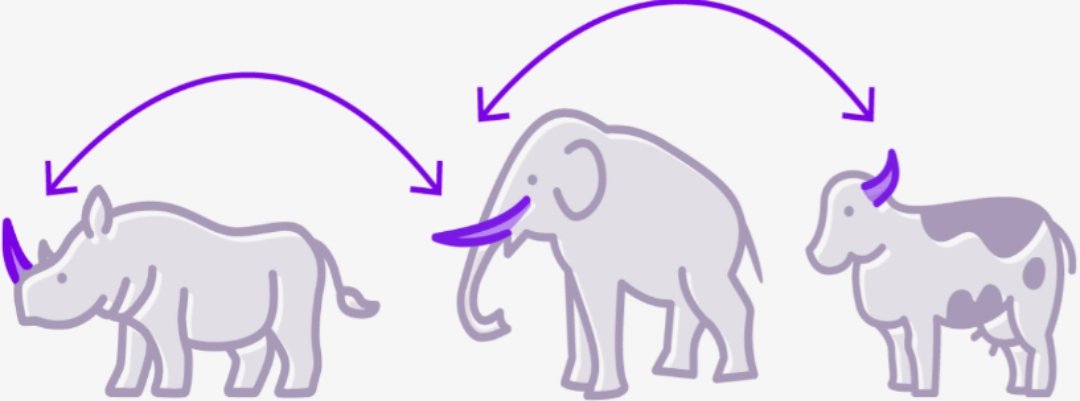
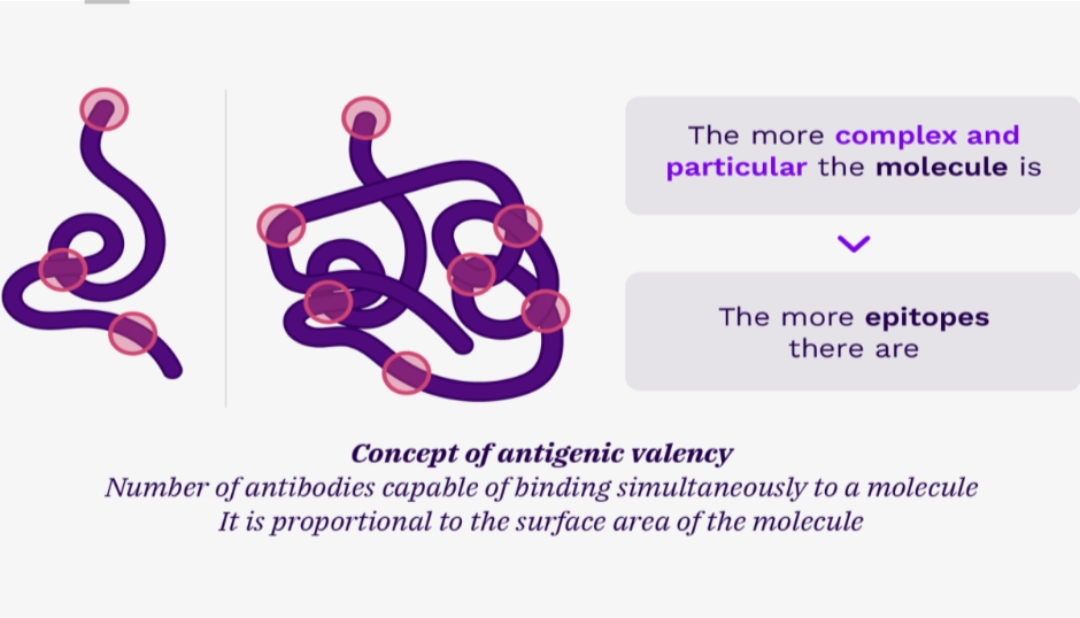
CROSS-REACTIVITYSince immunoreceptors recognize epitopes that are very small motifs within complex molecules, there may be epitope redundancy within very different molecules. |
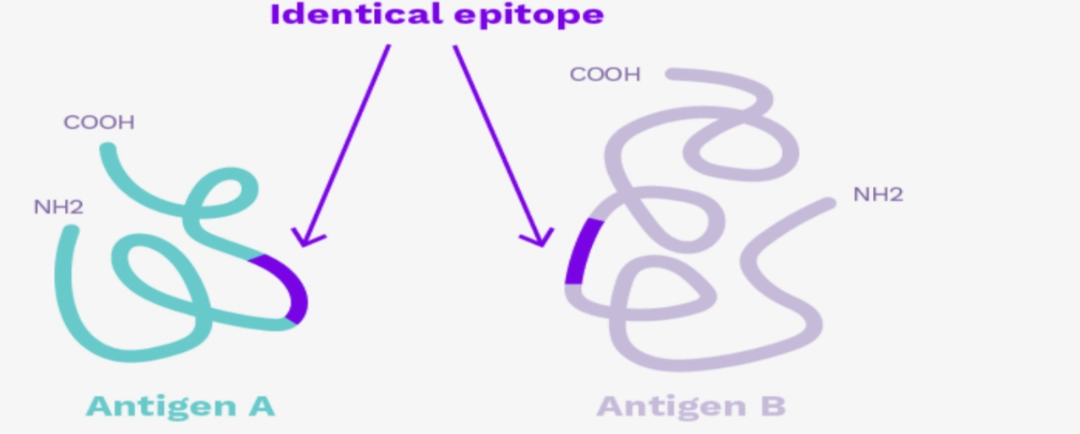 |
To go further:
This is particularly evident in cases of cross allergies, for example between kiwi and latex.
In transplantation in particular, there may be anti-HLA immunisation against HLA molecules that have never been encountered by the recipient. Most often, it is an anti-HLA immunisation against a molecularly close molecule which crosses with another HLA molecule.
The proximity between two HLA molecules can be quantified by the epitope difference between the two molecules.
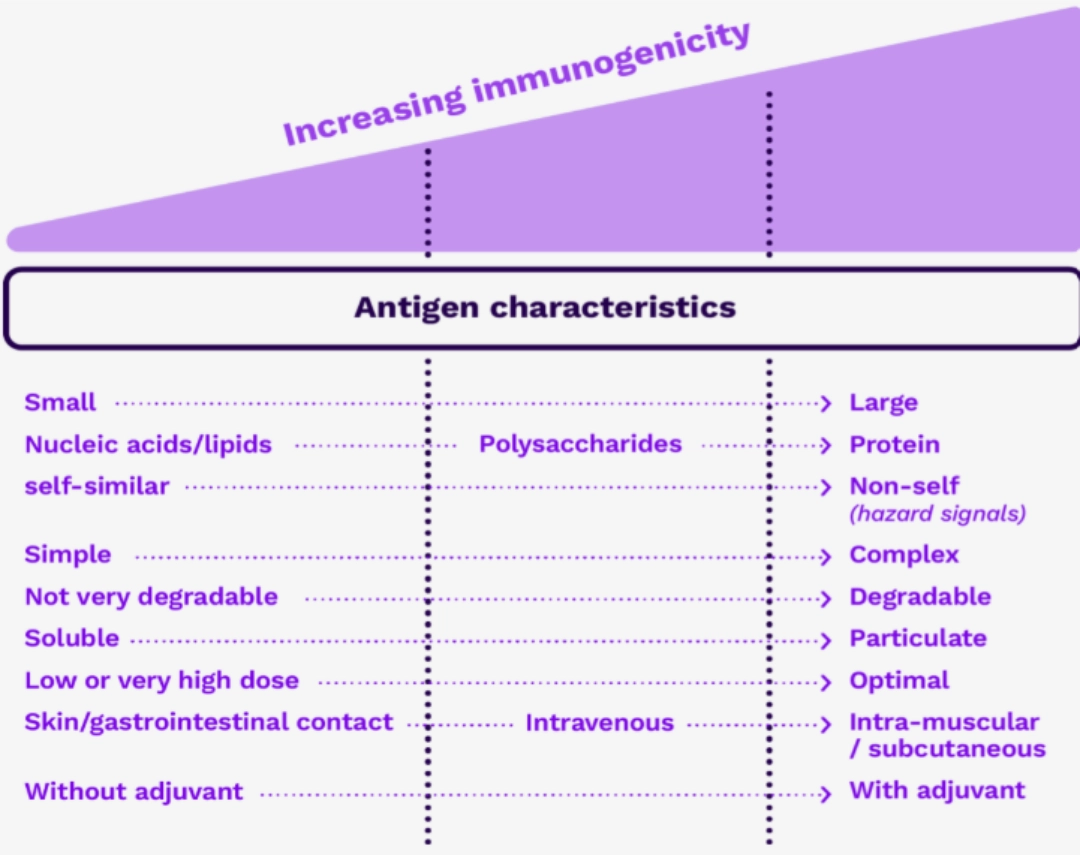
What needs to be remembered
Antigen is a molecular structure recognised by immunoreceptors. Depending on its characteristics, antigen may be more or less immunogenic. For transplantation, it is important to understand the concept of epitope and cross-reactivity: this explains why we can be immunised (have already produced antibodies) directed against molecules close to those already encountered. This is why it is essential to rigorously follow the anti-HLA immunisation of future organ recipients!