Immunology mechanism
Antigen Presentation
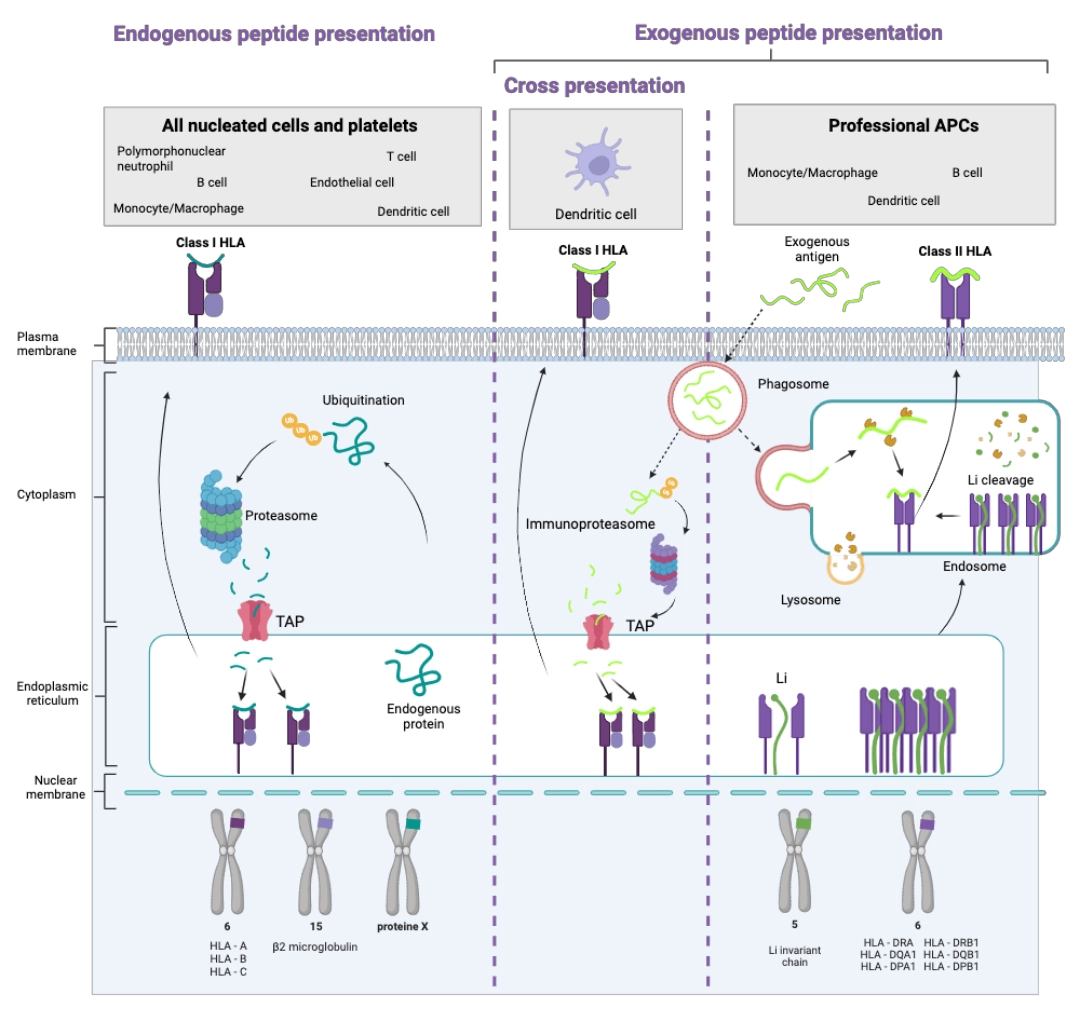
Antigen presentation is the process by which the body’s cells present, via their HLA molecules, antigen proteins in the form of short peptides to the T-cell TCRs
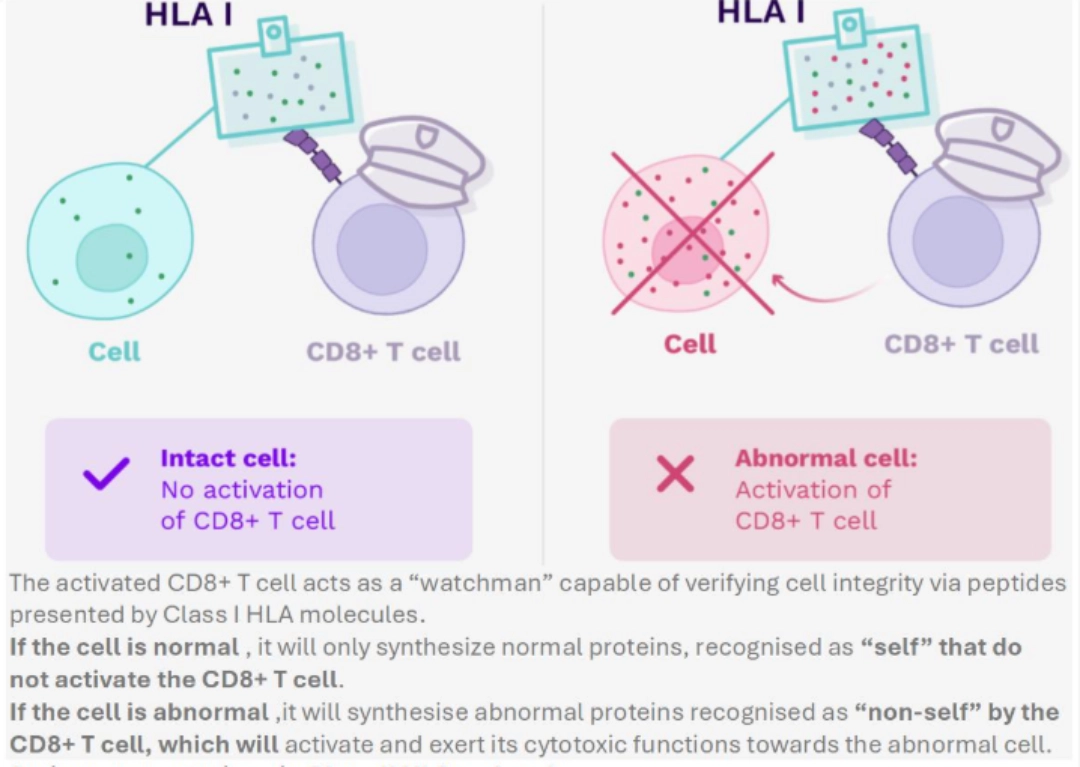
MechanismWhere?Antigen presentation via Class I HLA molecules
Antigen presentation via Class II HLA molecules
|
 |
When?
Antigen presentation via Class I HLA molecules
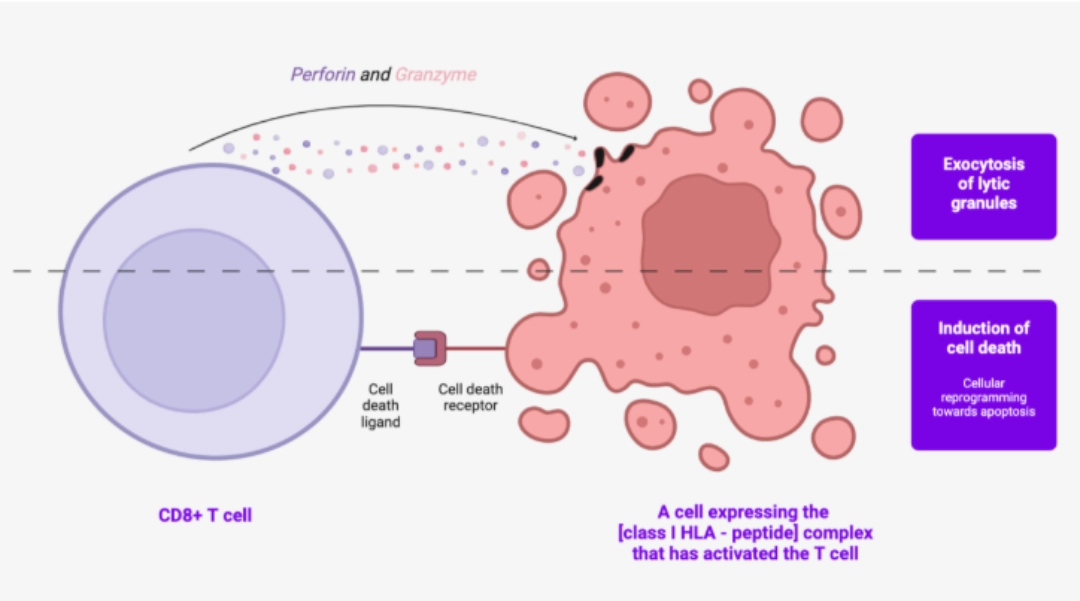
- Continuously, so that CD8+ T cells can recognise and then eliminate abnormal cells.
- The activated CD8+ T cell acts as a “watchman” capable of verifying cell integrity via peptides presented by Class I HLA molecules.
- If the cell is normal , it will only synthesize normal proteins, recognised as “self” that do not activate the CD8+ T cell.
- If the cell is abnormal ,it will synthesise abnormal proteins recognised as “non-self” by the CD8+ T cell, which will activate and exert its cytotoxic functions towards the abnormal cell.
Antigen presentation via Class II HLA molecules
- Continuously for professional antigen-presenting cells and thymic epithelial cells. Only in an inflammatory context for other cells.
How is this done?
After priming of peptides
Priming is the set of stages of degradation of a protein antigen, obligatory and prior to the embedding of the peptide in the groove of the HLA molecule.
Priming and antigen presentation of Class I HLA molecules
1% of all proteins produced by the cell are permanently sent for degradation via ubiquitin marking. This marking sends them to the proteasome where they will undergo the action of enzymes cleaving them into smaller peptides. These then pass from the cytosol to the endoplasmic reticulum using TAP pumps. Once in the endoplasmic reticulum, they can undergo the action of other proteolytic enzymes. At the end of these degradation and cleavage processes, we obtain in the endoplasmic reticulum a pool of small peptides of a few amino acids, derived from endogenous proteins.
Ultimately, the peptides that can be embedded in a Class I HLA molecule are:
- Endogenous
“Endogenous” and “self” are not synonymous
A protein is said to be endogenous if it is synthesised by the cell that will present it. However, a cell can synthesise different “self” proteins in a non-physiological context (stress, particularly viral infection, malignant degeneration, etc.).
An endogenous protein synthesized by the body is therefore not necessarily “of itself”! In contrast, all endogenous proteins (“self” and “non-self”) are presented via Class I HLA molecules.
- Short
Mostly 9 amino acids, i.e., nonamers.
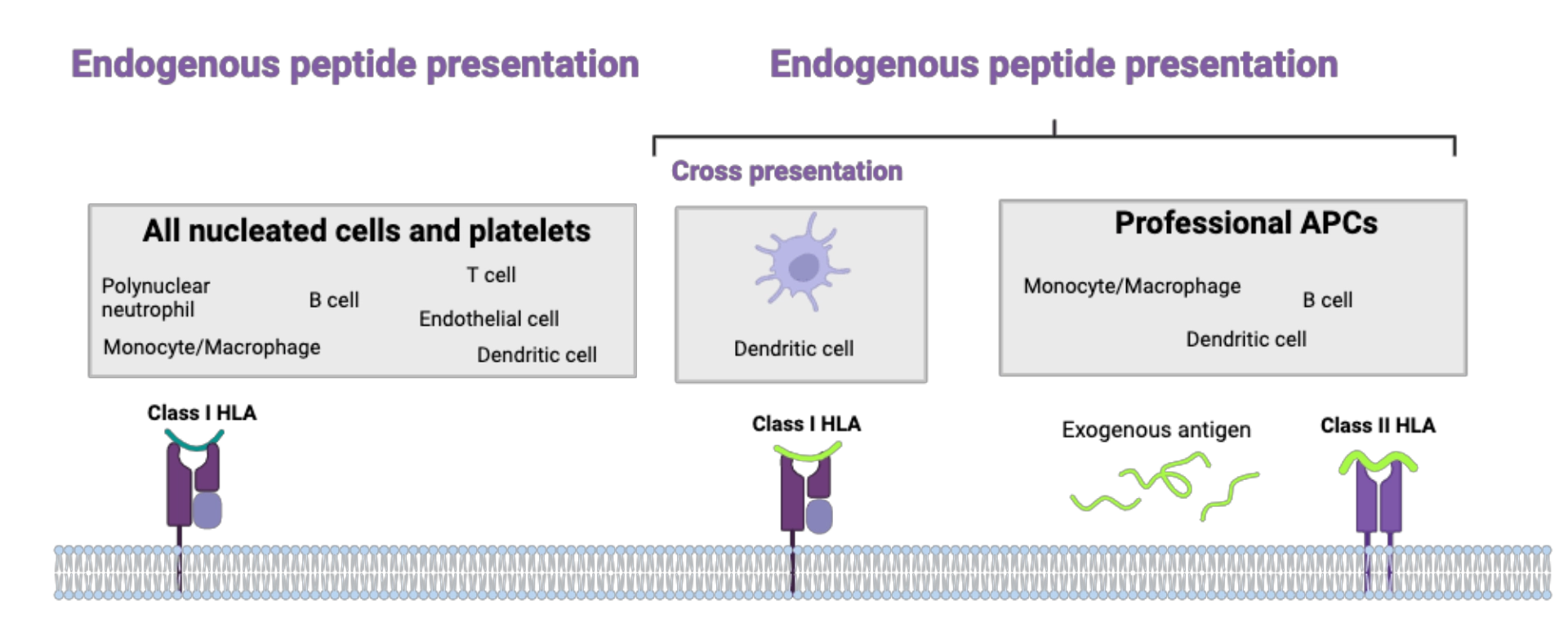
Once loaded with a peptide, Class I HLA molecules are expressed on the surface of all nucleated cells and platelets. Complexes [peptide - Class I HLA molecule] can interact with the TCR of CD8+ T cells and the KIRs of NK cells.
To go further
Priming and antigen presentation of Class II HLA molecules
The peptide pocket of Class II HLA molecules forming in the endoplasmic reticulum is protected by the invariant Li chain to prevent peptides present in the endoplasmic reticulum from becoming embedded there. As soon as the complex [Li chain – Class II HLA molecule] is formed, it is transported to the endosome. By carrying out its role of recycling exogenous and transmembrane proteins, the endosome becomes the loading site, into Class II HLA molecules, of peptides of 12 to 25 amino acids emanating from degradation by lysosomal enzymes. The invariant Li chain is also degraded to make room for the peptide.
The complex [peptide - Class II HLA molecule] is expressed, in the basal state, on the surface of dendritic cells, B cells, macrophages and thymic epithelial cells. During an inflammatory response, certain other cells (including endothelial cells and T cells) can also express Class II HLA molecules.
The particular case of cross-presentation carried out by the dendritic cell
The dendritic cell is the only one able to carry out cross-presentation, that is to say able to present peptides resulting from the degradation of exogenous proteins via Class I HLA molecules. It is a major characteristic of this cell, which makes it responsible for the link between innate immunity and adaptive immunity. Indeed, it is thanks to this cross-presentation that the dendritic ell can activate CD8+ T cells to induce a cellular reaction towards antigens not expressed in the dendritic cell.
Consequences
Unlike the B cell which recognises any native antigen thanks to its BCR, the T cell is incapable of recognizing a native antigen. The antigen priming and presentation mechanisms are therefore strictly obligatory: without them, none of the mechanisms in which T cells are involved could take place!
What needs to be remembered
| Nucleated cells & platelets | Dendritic cell | B cell | Macrophage | |
|---|---|---|---|---|
| HLA molecule expressed at basal state |
Class I | Class I Class II |
Class I Class II |
Class I Class II |
| Origin of the peptide presented |
Endogenous | Endogenous & Exogenous | Exogenous Endogenous |
Exogenous Endogenous |
| Interaction with T-cell TCR |
CD8+ | CD8+ CD4+ |
CD8+ CD4+ |
CD4+ |
| Destruction of infected, abnormal cells | Thymic education of CD8+ and CD4+ T cells | Destruction of infected B-cells CD4+ T-cell/B-cell cooperation |
Destruction of infected macrophages T-cell (CD4+ Th1)/macrophage cooperation (e.g., tuberculosis) |
|
| Function of antigen presentation |
Peripheral activation of CD8+ and CD4+ T cells | |||
| Destruction of infected, abnormal dendritic cells |
T cell activationT cell activation refers to all the steps necessary for the transformation of a naive T cell into a T cell capable of exercising its functional properties. |
 |
T cell activation refers to all the steps necessary for the transformation of a naive T cell into a T cell capable of exercising its functional properties.
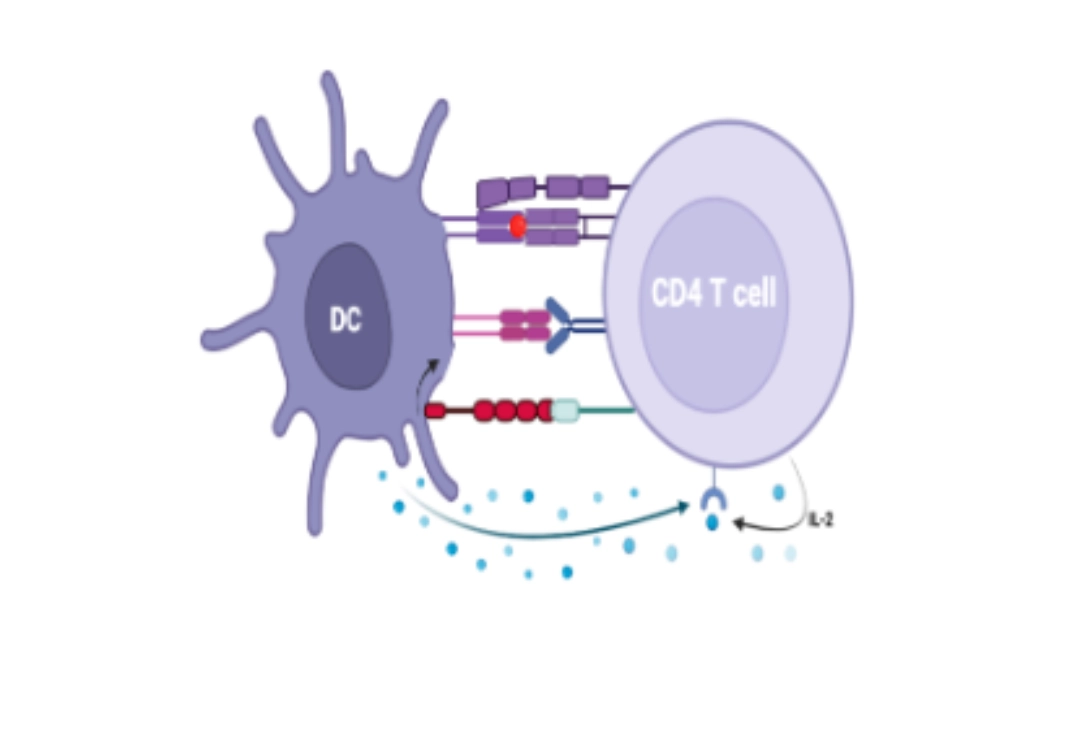
MECHANISMWhere?In the T cell zone of the secondary lymphoid organs, which constitutes a zone of concentration of antigens:
|
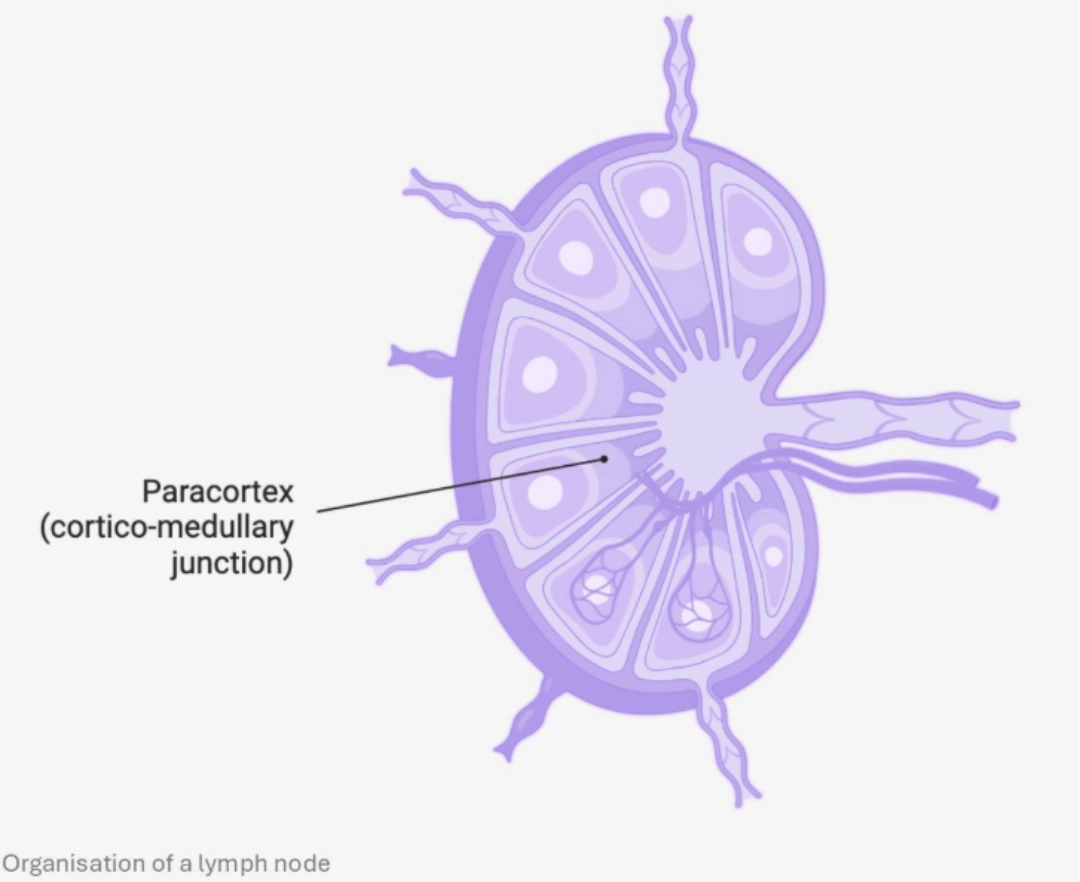 |
When?After undergoing positive and negative selection in the thymus eliminating ineffective and self-reactive clones respectively (see T cell ontogeny), T cells circulate in the bloodstream and enter secondary lymphoid organs. They scout the dendritic cells, guided by the network of reticular fibroblastic cells. If a T cell recognises the complex [HLA-peptide molecule] corresponding to its TCR on the surface of a dendritic cell, this constitutes its first activation signal. |
 |
To go furtherHow is this done?Three activation signals of naive T cells can only be delivered by dendritic cells. |
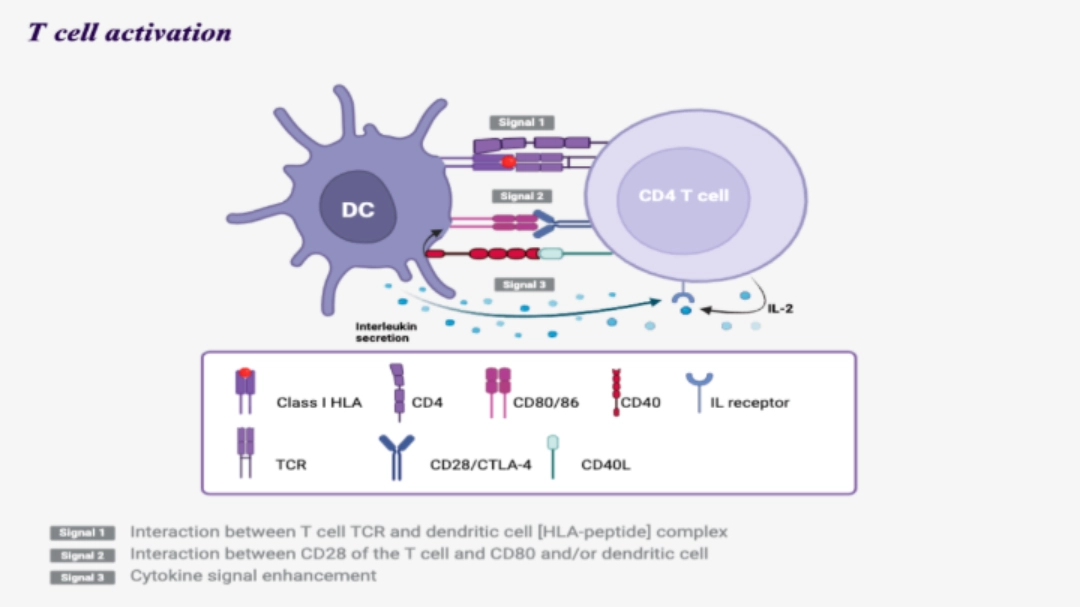 |
Signal 1
- Interaction between the T cell TCR and the [HLA molecule - peptide] complex of the dendritic cell.
- Ensures the specificity of T cell activation
- This recognition is stabilised by the interaction of CD4 or CD8 of the T cell with the HLA molecule of the dendritic cell, Class II or I, respectively. Following this interaction, CD3 associated with the TCR transduces a signal via the ITAM motifs present intracellularly.
Signal 2
- Interaction between CD28 of the T cell and CD80 and/or CD86 of the dendritic cell.
- Avoids anergy
- This interaction amplifies and complements the intracellular activation signals from the [TCR-CD3] complex, which will allow the secretion of IL-2 by the dendritic cell and the expression of an IL-2R receptor by the T cell. Without this second signal and IL-2, the T cell would be anergic.
Signal 3
- Via cytokines present in the microenvironment.
- Allows clonal expansion of the T cell
Allows functional differentiation of CD4+ and CD8+ T cells
- Cytokines are secreted mainly by dendritic cells, but also by other cells in the microenvironment. They allow the functional differentiation of lymphocytes into cytotoxic T cells for CD8+ T cells or helper T cells for CD4+ T cells, as well as the initiation of clonal proliferation.
Stopping activation
To escape the interaction with the dendritic cell, the T cell expresses after 2 to 3 days the CTLA-4 molecule (Cytotoxic T Lymphocyte Antigen 4, or CD152), which is more affinity for the CD80 and CD86 molecules than the CD28.
Unlike the interaction [CD28-CD80/86], the interaction [CTLA-4-CD80/86] does not generate an activation signal to the T cell. The CTLA-4 expression will therefore stop the interaction with the dendritic cell , which allows the T cell to:
- either leave the secondary lymphoid organ to proliferate into functional clones directed against the antigen;
- or head towards the T/B interface zone of the secondary lymphoid organs to contribute to the activation of B cells.
Consequences
The activation of T cells will lead to a clonal expansion , which increases 1,000 to 10,000 times the number of T cells specific for the complex [HLA-peptide molecule] initiating this activation. The vast majority of activated T cells leave the secondary lymphoid organs to exert their effector actions in the tissues, towards the cells which present the same complex [HLA-peptide molecule] on their surface.
The effector functions of T cells are mainly:
For CD4+ T cell: co-activator signals (auxiliary or helper lymphocytes)
The previously activated CD4+ T cell interacts with the B cell to allow it to fully activate after it has recognised an antigen via its BCR: It is the T/B-cell cooperation (see B cell activation). CD4+ T cells also integrate signals from the environment, and respond to them by in turn synthesising cytokines, which will guide the immune response. Depending on the cytokines that the lymphocyte secretes, we distinguish different profiles, the best known of which are the Th1, Th2, and Th17 profiles. CD4+ T cell profiles will not be further covered here.
IMMUNOSUPPRESSANTS THAT ACT ON THE ACTIVATION OF T CELLS
| Target | Therapeutic class |
|---|---|
| Signal 1 TCR interaction - [HLA molecule - peptide] |
Calcineurin inhibitors |
| Signal 2 Co-stimulation signal |
Corticosteroids, CTLA4-Ig fusion protein |
| Signal 3 Action of IL-2 |
Anti-CD25 monoclonal antibodies (IL-2 receptor), mTOR inhibitors |
| Clonal proliferation blockade | Antimetabolites acting on purine metabolism* |
* These substances have a more marked cytostatic effect on lymphocytes than on other cells due to the dependence of T and B cells on the de novo synthesis of purines, while other cell types can use supplementary metabolic pathways.
What needs to be remembered
|
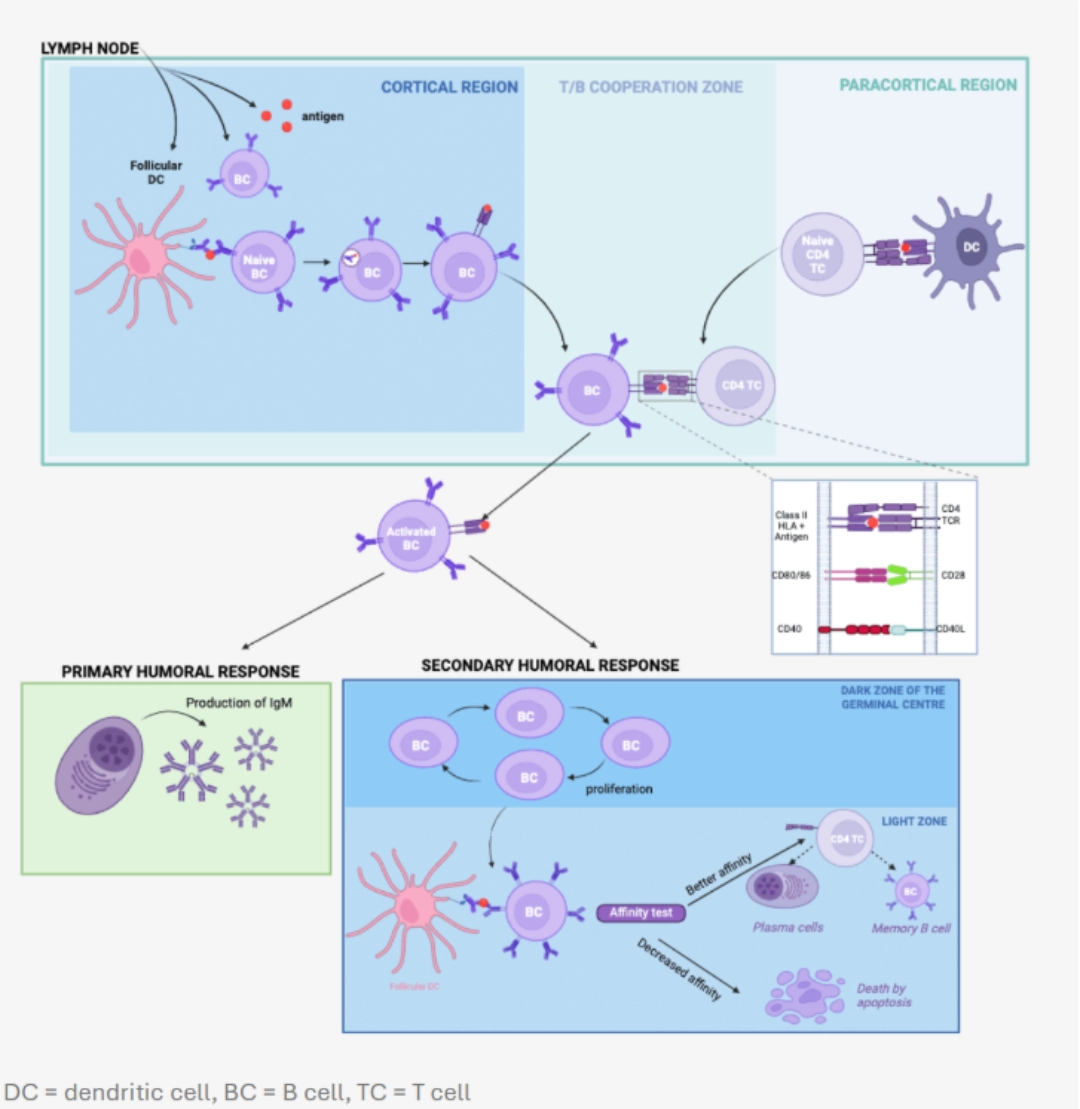
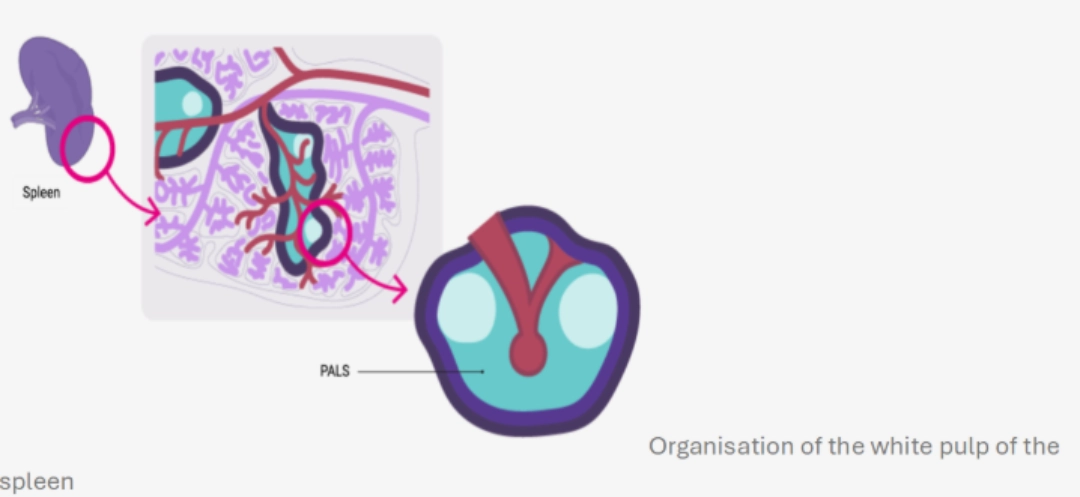
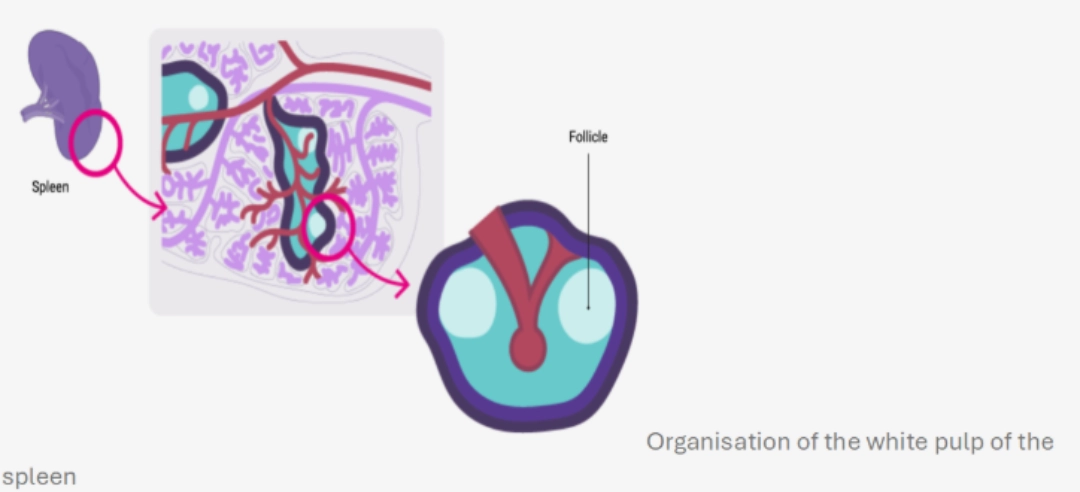
MechanismWhere?In the B cell zone of the secondary lymphoid organs, which constitutes a zone of concentration of antigens:
|
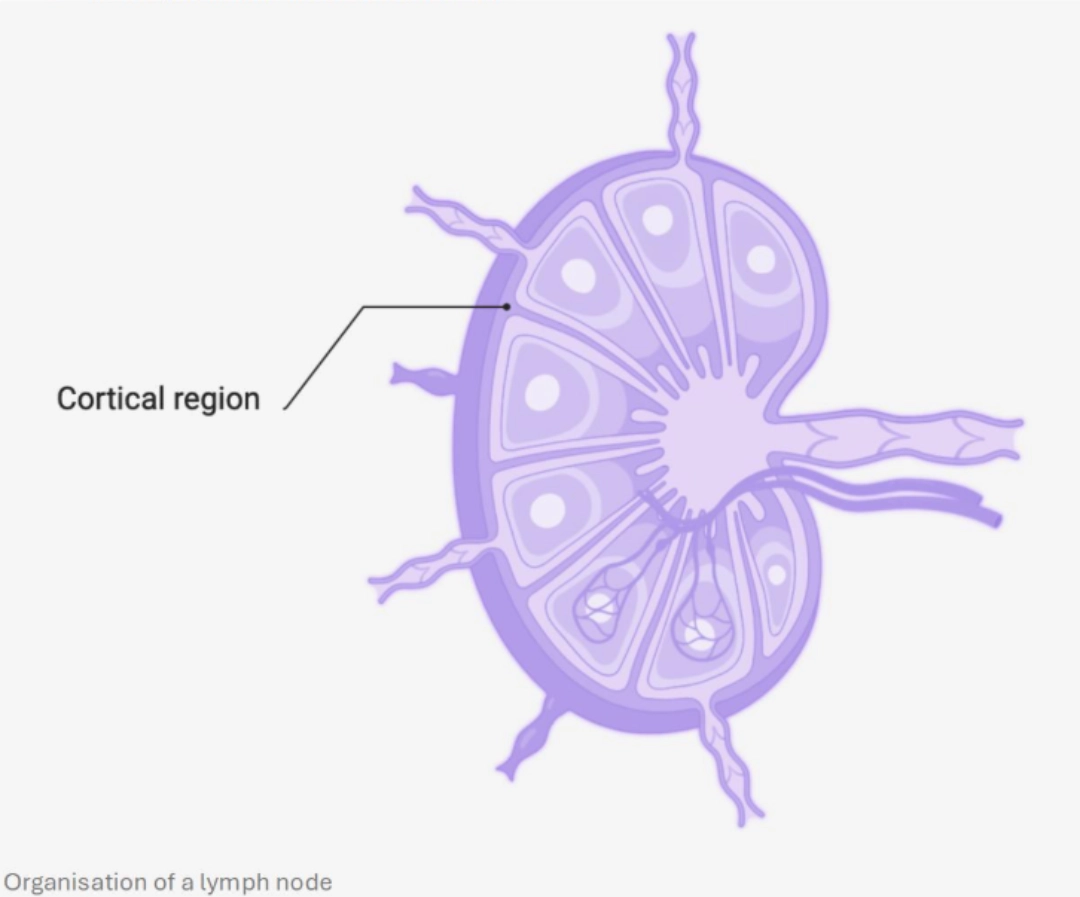 |
When?After having undergone central selection in the bone marrow, then peripheral in the blood and in the spleen in order to eliminate auto-reactive B cells (see B cell ontogeny), B cells circulate in the bloodstream and enter secondary lymphoid organs. They are looking for the antigen that could be recognised by their BCR. If a B cell recognises a native antigen corresponding to its BCR, this constitutes its first activation signal. |
 |
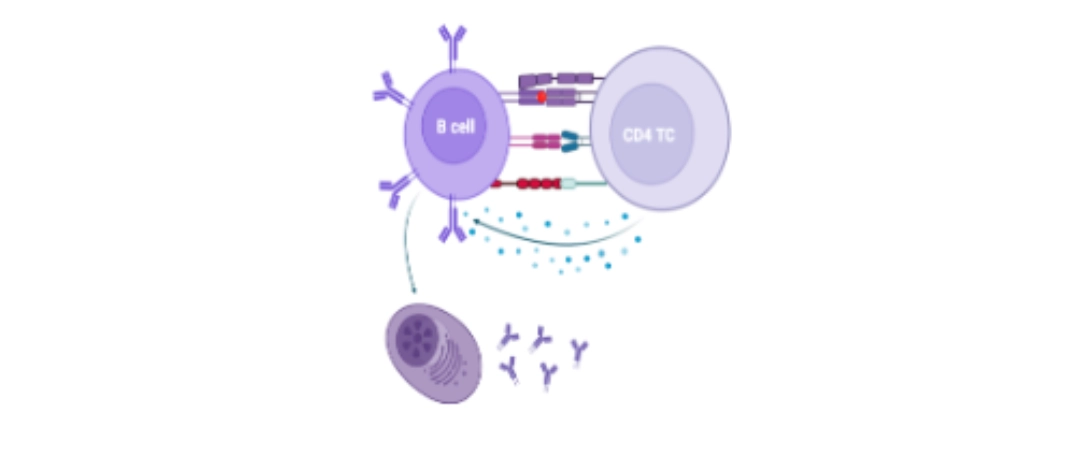
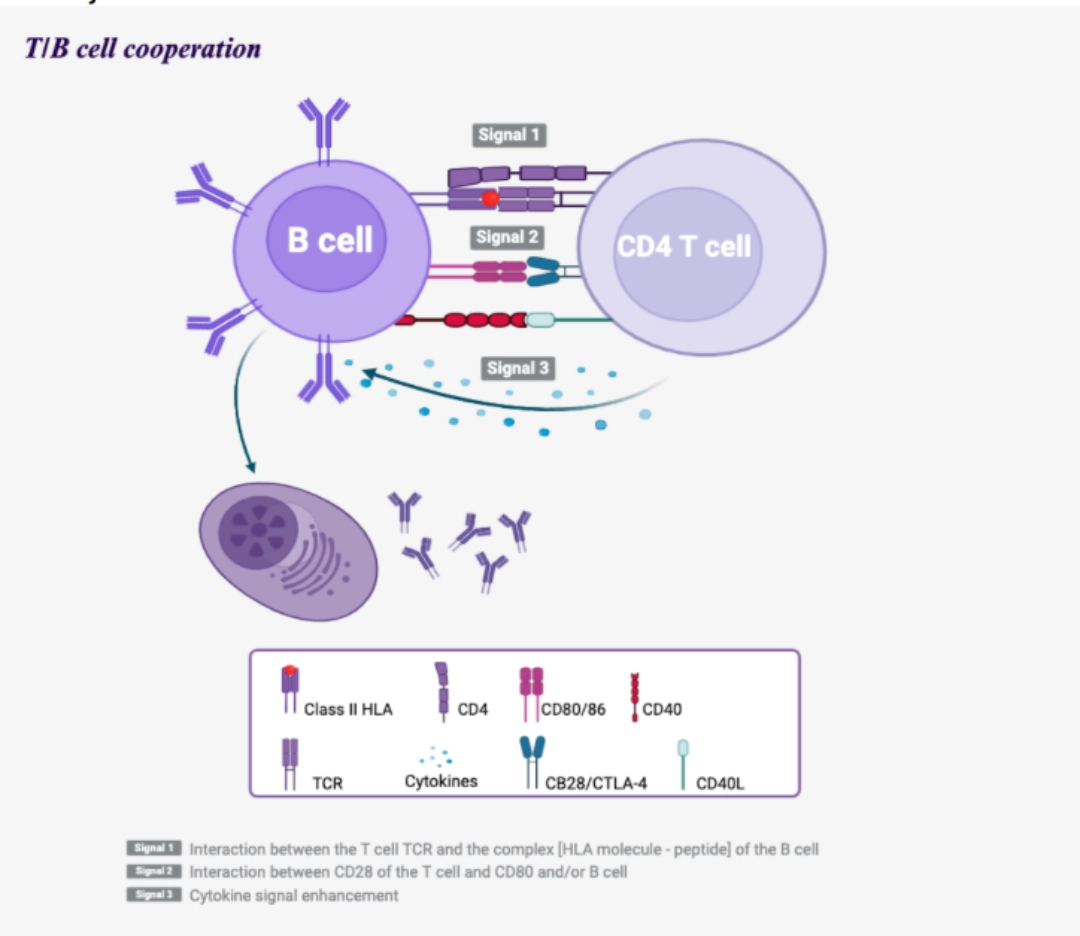
Signal 1
Recognition of the native antigen by the BCR of the naive B cell, then internalisation.
Ensures the specificity of B-cell activation
The native antigen is transported to the secondary lymphoid organs in the form:
- soluble;
- of a circulating immune complex;
- of immune complex attached to follicular dendritic cells or macrophages present in secondary lymphoid organs.
The engagement of at least two BCRs of the same B cell results in the formation of a bridge necessary for signal transduction by CD79 associated with the BCRs. The downstream pathway is initiated by the phosphorylation of the ITAM motifs of the CD79 chains, which will induce the expression of chemokine receptors on the surface of the B cell, and thus promote the migration of the B cell into the T/B cooperation zone of the secondary lymphoid organs.
In parallel, the antigen is internalised and processed to be presented via a Class II HLA molecule.
Reminder
The B cell is not capable of cross-presentation.
Signal 2
CONSEQUENCES
Interactions between the CD4+ T cell and B cell promote:
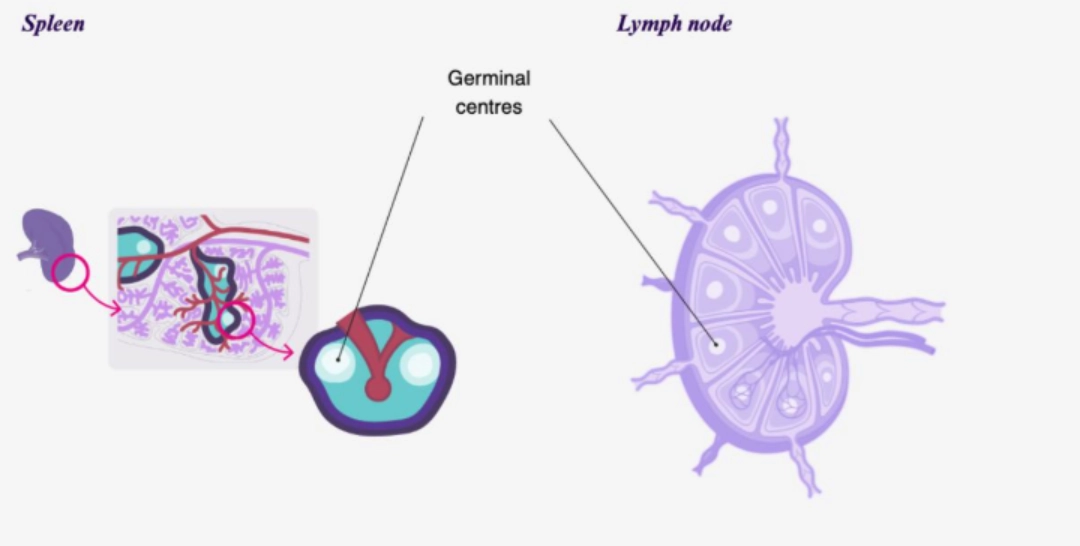
education of germinal centres
- The germinal centre is a substructure dedicated to the generation of B cell clones expressing immunoreceptors (BCR) with very high affinity for an antigen. A germinal centre develops at the expense of the follicular zone, whether in the spleen or in the lymph node. The appearance of a germinal centre signals the transition from a primary follicle to a secondary follicle.
Somatic hypermutation of the BCR
The B cell clone engaged in the formation of a germinal centre proliferates and undergoes somatic hypermutations of the genes encoding the BCR variable region thanks to an enzyme called AID (Activation-Induced Cytidine Deaminase). The follicular dendritic cells present in the environment present the antigen to them continuously to test the affinity of the new receptor towards the antigen :
if the new BCR has better affinity, then the B cell survives and differentiates into a plasma cell or a memory B cell;
if, on the contrary, the BCR has a lower affinity, then the clone degenerates.
Class switching
B cells initially express only membrane IgM or IgD (see B cell ontogeny). The interaction with the CD4+ T cell, as well as the cytokine environment within the secondary follicle modifies the cellular programming of the B cell (notably the splicing programs), which makes it possible to modify the isotype of the immunoglobulins secreted by the plasma cell (IgM ➔ IgG, IgA or IgE).
| Differentiation of B cells into long-lived plasma cells |
|
IMMUNOSUPPRESSANTS ACTING ON THE ACTIVATION OF B CELLS
In organ transplantation, the inhibition of B cell activation is mainly achieved indirectly, through the inactivation of helper T cells. Drugs specifically targeting the B cell lineage are anti-CD20 monoclonal antibodies and proteasome inhibitors that deplete B cells and plasma cells, respectively.
What needs to be remembered
|