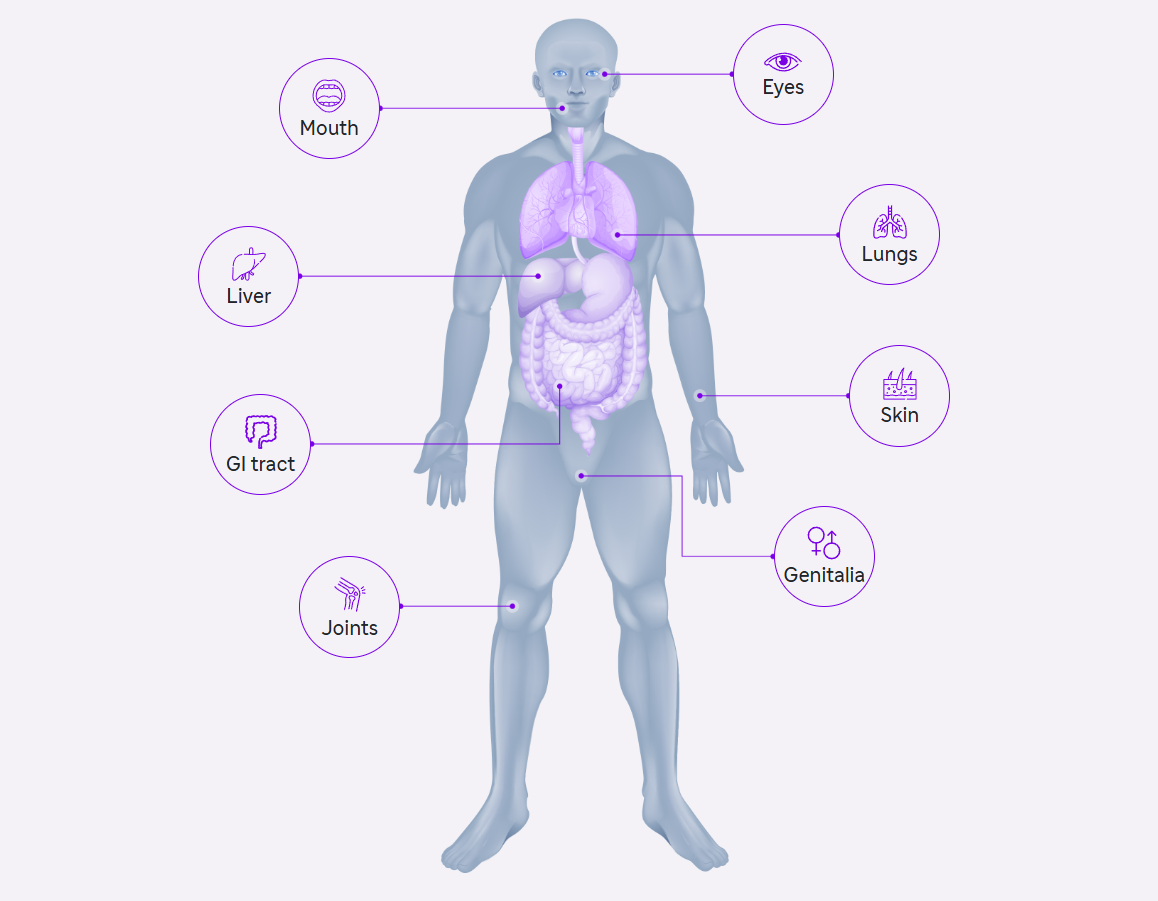
Effect of cGVHD
- Immunological activation leads to ocular surface inflammation and lacrimal gland fibrosis in oGvHD.
- Self-reactive T cells (CD4+ and CD8+) from the donor persist due to defective central and peripheral tolerance mechanisms.
- T cell-mediated immune response targets host antigens, including major (MHC) and minor (miHAG) histocompatibility antigens, driven by differences in host and donor antigen expression, such as HLA mismatch and polymorphic minor histocompatibility antigens.
- Imbalance between effector and regulatory T cell functions triggers inflammatory cascades.
- Donor T cells play a predominant role in systemic and ocular disease orchestration, although B cells and antigen-presenting cells are also involved.
- In oGvHD, APC activation, donor T cell differentiation, proliferation, and activation, along with B cell activation and pro-inflammatory cytokine release, induce and maintain ocular surface inflammation and activate fibroblasts and dendritic cells in the lacrimal gland, ultimately leading to lacrimal tissue fibrosis.
- Tissue damage in oGvHD extends beyond the ocular surface and lacrimal gland, involving ocular adnexa, with Meibomian gland and ocular surface damage correlating with each other.
Pathophysiology of ocular GvHD
- Immunological activation leads to ocular surface inflammation and lacrimal gland fibrosis in oGvHD.
- Self-reactive T cells (CD4+ and CD8+) from the donor persist due to defective central and peripheral tolerance mechanisms.
- T cell-mediated immune response targets host antigens, including major (MHC) and minor (miHAG) histocompatibility antigens, driven by differences in host and donor antigen expression such as HLA mismatch and polymorphic minor histocompatibility antigens.
- Imbalance between effector and regulatory T cell functions triggers inflammatory cascades.
- Donor T cells play a predominant role in systemic and ocular disease orchestration, although B cells and antigen-presenting cells are also involved.
- In oGvHD, APC activation, donor T cell differentiation, proliferation, and activation, along with B cell activation and pro-inflammatory cytokine release, induce and maintain ocular surface inflammation and activate fibroblasts and dendritic cells in the lacrimal gland, ultimately leading to lacrimal tissue fibrosis.
- Tissue damage in oGvHD extends beyond the ocular surface and lacrimal gland, involving ocular adnexa, with Meibomian gland and ocular surface damage correlating with each other.
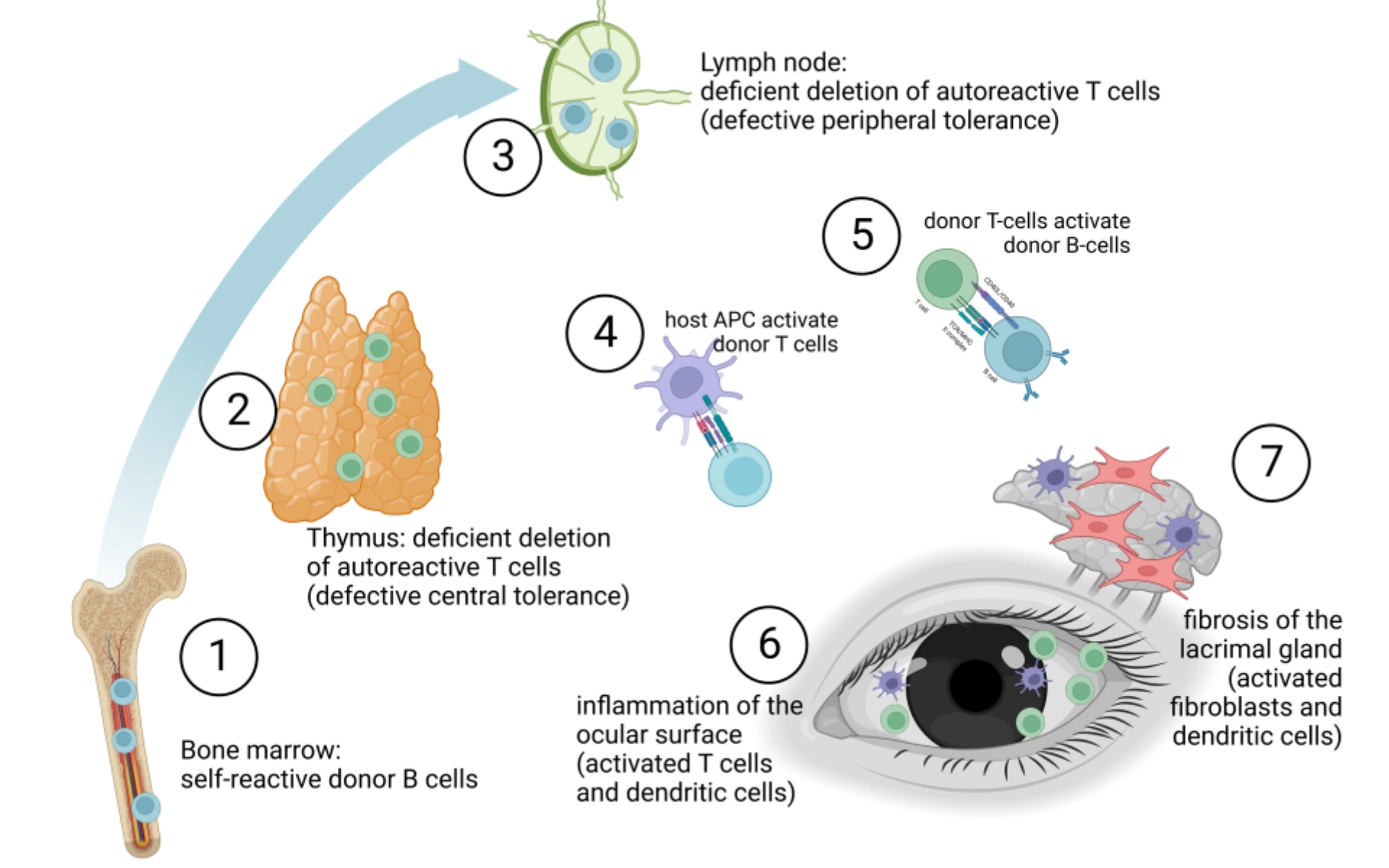
This image was obtained from an open-access article distributed under the terms of the Creative Commons Attribution License(CC-BY). APC: Antigen-Presenting Cells; CD4+:
Cluster of Differentiation 4; CD8+: Cluster of Differentiation 8; HLA: Human Leukocyte Antigen; MHC: Major Histocompatibility Complex; miHAG: Minor Histocompatibility Antigens; oGvHD: Ocular Graft-versus-Host Disease
1. Tappener C et al. Front Med (Lausanne). 2023.10;1133381.
Diagnosis
Characteristics of cGvHD: Eyes
Eyes: Distinctive KCS
- New onset of dry, gritty or painful eyes
- Cicatricial conjunctivitis
- KCS
- Confluent areas of punctate keratopathy
Eyes: Other2,3
- Photophobia
- Periorbital hyperpigmentation
- Blepharitis (i.e., erythema and edema of eyelids, telangiectasia of eyelid margin)
- Scoring of cGvHD: Eyes4
Eyes4
| SCORE 0 | SCORE 1 | SCORE 2 | SCORE 3 | |
| EYES Keratoconjunctivitis sicca (KCS) confirmed by ophthalmologist: ☐ Yes ☐ No ☐ Not examined |
☐ No symptoms | ☐ Mild dry eye symptoms not affecting ADL (requirement of lubricant eye drops ≤ 3 x per day) |
☐ Moderate dry eye symptoms partially affecting ADL (requiring lubricant eye drops > 3 x per day or punctal plugs), WITHOUT new vision impairment due to KCS |
☐ Severe dry eye symptoms significantly affecting ADL (special eyeware to relieve pain) OR unable to work because of ocular symptoms OR loss of vision due to KCS |
| ☐ Abnormality present but explained entirely by non-GVHD documented cause (specify): | ||||
‡To be completed by specialist or trained medical providers.
cGvHD, chronic graft-versus-host disease; KCS, keratoconjunctivitis sicca; ADL, activities of daily living.
- Nassiri N et al. J Ophthalmic Vis Res. 2013;8(4):351-358;
- Screening eyes for chronic GvHD. BetheMatchClinical.org. Accessed April 11, 2022. https://bethematchclinical.org/post-transplant-care/chronic-gvhd/eyes/;
- Smith Knutsson E et al. Acta Obstet Gynecol Scand. 2018;97(9):1122-1129. doi:10.1111/aogs.13366;
- Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001.
The figures are included from the article Nair S et al. Indian J Ophthalmol. 2021 May;69(5):1038-1050; which is under the copyright license of CC-BY-NC that permits the non-commercial use.
Management
Treatment of ocular cGvHD1,2
- Lubrication: Intensive lubrication for dry and inflamed ocular surface is an essential parameter for the treatment of ocular cGvHD. Several artificial tears, viscous eye drops and ointments are available with limited data.
- Topical anti-inflammatory agents: Topical corticosteroids have been used with limited data on efficacy. However, cyclosporine eye drops have been used to treat refractory eye disease.
- Increase teat and mucin production: Oral muscarinic agonist such as pilocarpine or cevimeline can be used as an adjuvant treatment approach. Other medication include secretagogue eye drop (diquafosol or rebamipide)
- Reducing train drainage: Using collagen or silicone punctual plugs can be inserted into lacrimal ducts
- Scleral lenses: Using scleral lenses can be pivotal in reducing ocular symptoms including ocular pain and promote visual activity by forming a uniform surface
- Systemic treatment: High dose corticosteroids (methylprednisolone 1 mg/kg) is the first line treatment with options for second-line and third-line treatments
- Surgical management of complications
- Lid care/warm compress/humidified environment
- Bandage contact lens (used with caution)

- GvHD related Dry Eye Disease is often overlooked due to lack of subjective symptoms and reliable testing in children. Schirmer’s test is difficult for children because it is painful and take 5 minutes to complete. Routine ophthalmological screening should be done periodically post-HSCT.
- Although severe ocular sicca is uncommon in children with cGvHD, measured tear production is reduced, and surveillance for keratoconjunctivitis sicca is necessary.
- Ocular sicca generally responds to ancillary measures in conjunction with systemic immunosuppression.
- Experience is limited and dosing is not established for many of the topical and oral medications for ocular GvHD.
cGvHD, chronic graft-versus-host disease; GvHD, graft-versus-host disease; HSCT, hematopoietic stem-cell transplantation.
- Tappeiner C et al. Front Med. 2023;10:1133381.
- Carpenter PA et al. Biol Blood Marrow Transplant. 2015;21(7):1167-87.
Effects
Pathophysiology of cGvHD: Mouth1
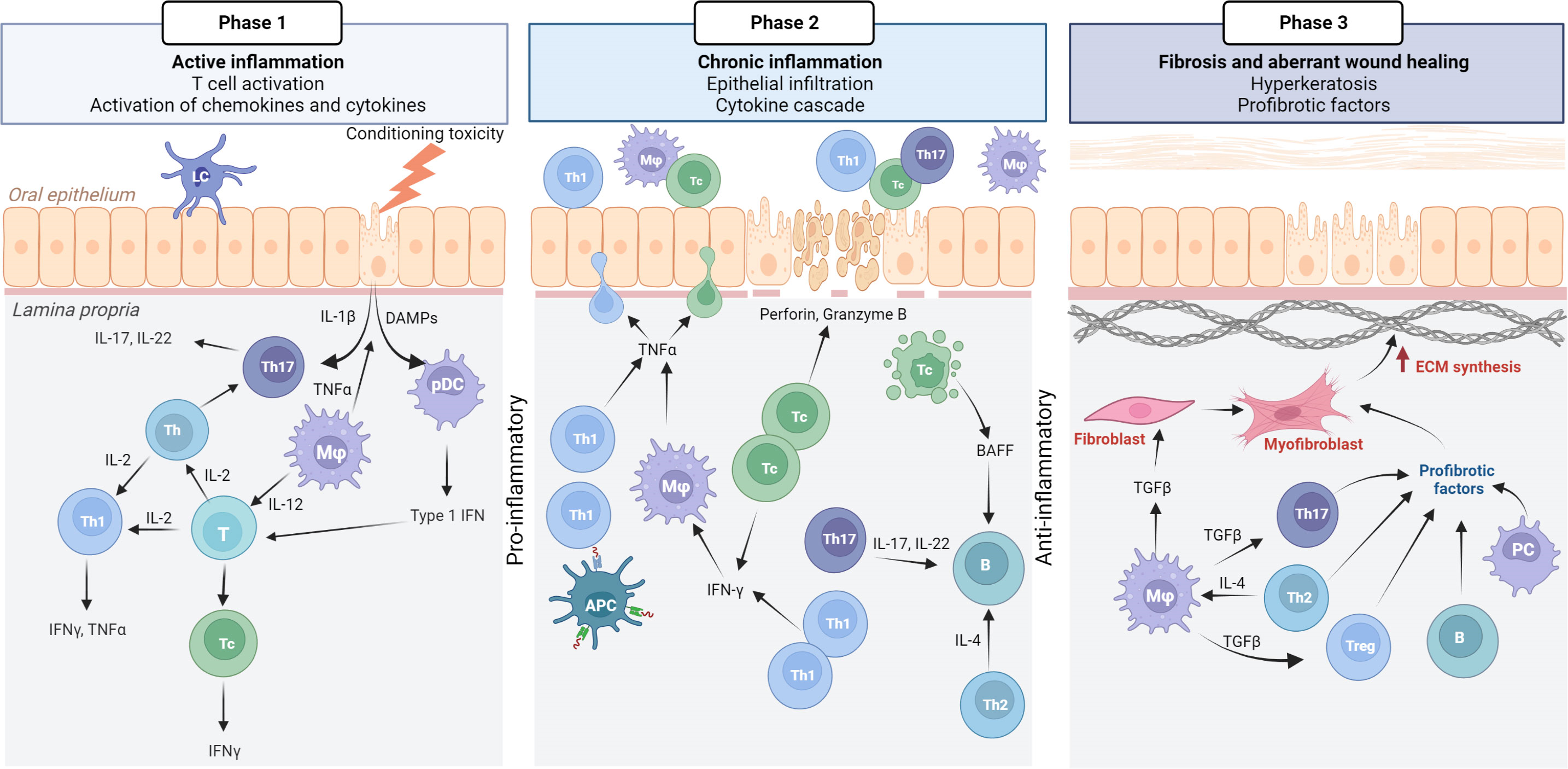
Phase 1: Active inflammation is initiated by conditioning toxicity, resulting in mucous membrane disruption, aGvHD, and viral reactivation. DAMPs, chemokines, and cytokines are released from endothelial, epithelial, and innate immune cells, leading to T cell activation. Initially, Th17 cells support epithelial maintenance and acute inflammation.
Phase 2: Plasmocytic dendritic cells (pDCs) with type 1 IFN secretion attract Th1/Tc1 response, which drives the chronic inflammatory phase.
Phase 3: IFN-γ secretion promotes activated macrophages (M1) with TNF-α. Tc cells secrete perforin and granzyme B, inducing apoptosis, which leads to the secretion of BAFF, affecting B cells, although this requires further confirmation in oral mucosal cGvHD pathophysiology. Many cell types, including anti-inflammatory macrophages, secrete TGF-β as the inflammatory response transitions to a fibrotic stage.
Phase 1 cGvHD
|
|
Phase 2 cGvHD
|
|
Phase 3 cGvHD

|
|
aGvHD, acute graft-versus-host disease; B cells, bursa-derived cells; cGvHD, chronic graft-versus-host disease, T cells, thymocyte cells.
- Tollemar V et al. Front Immunol. 2023. 14:1151493
These images was obtained from an open-access article distributed under the terms of the Creative Commons Attribution License (CC-BY)
Diagnosis
Characteristics of cGvHD: Mouth
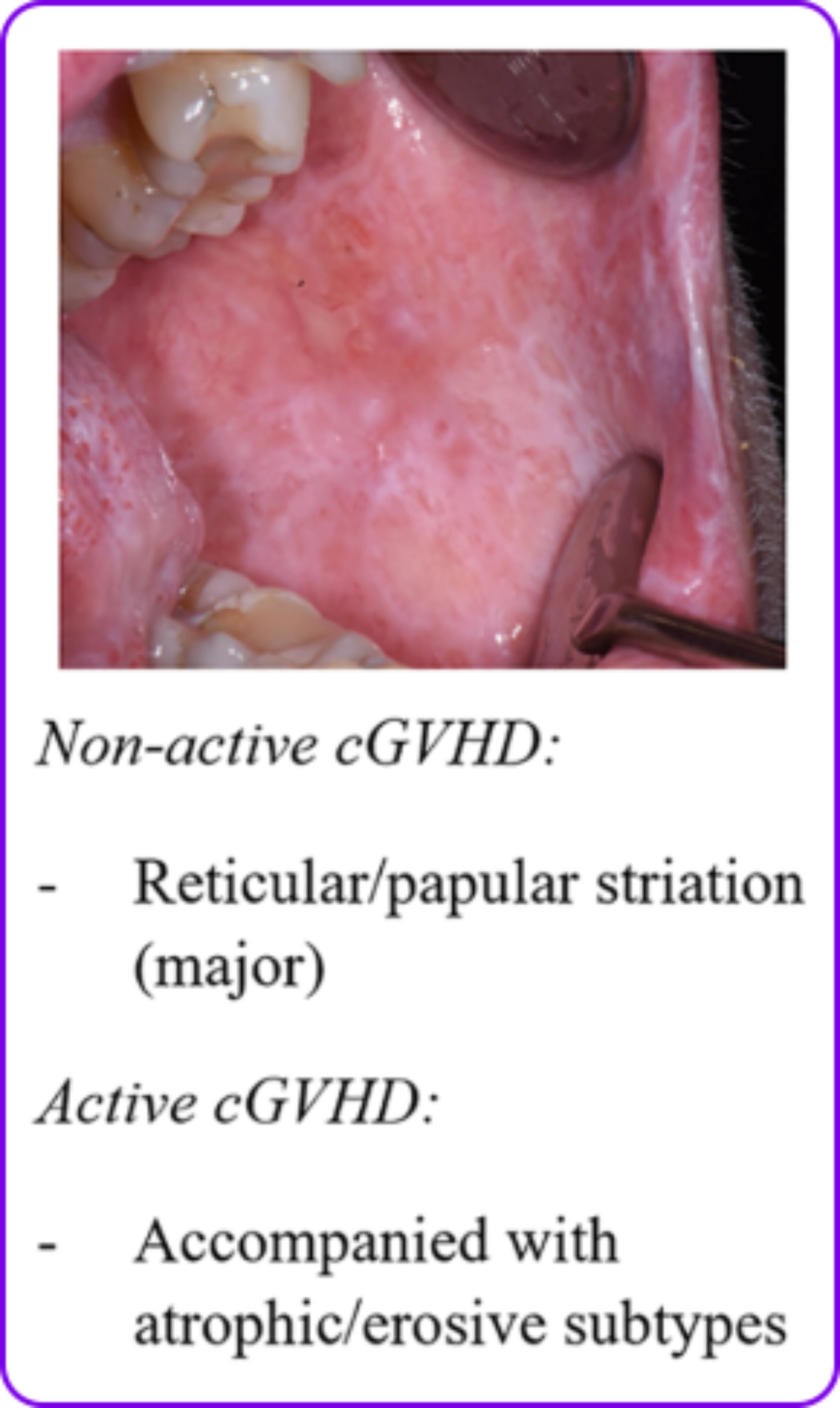
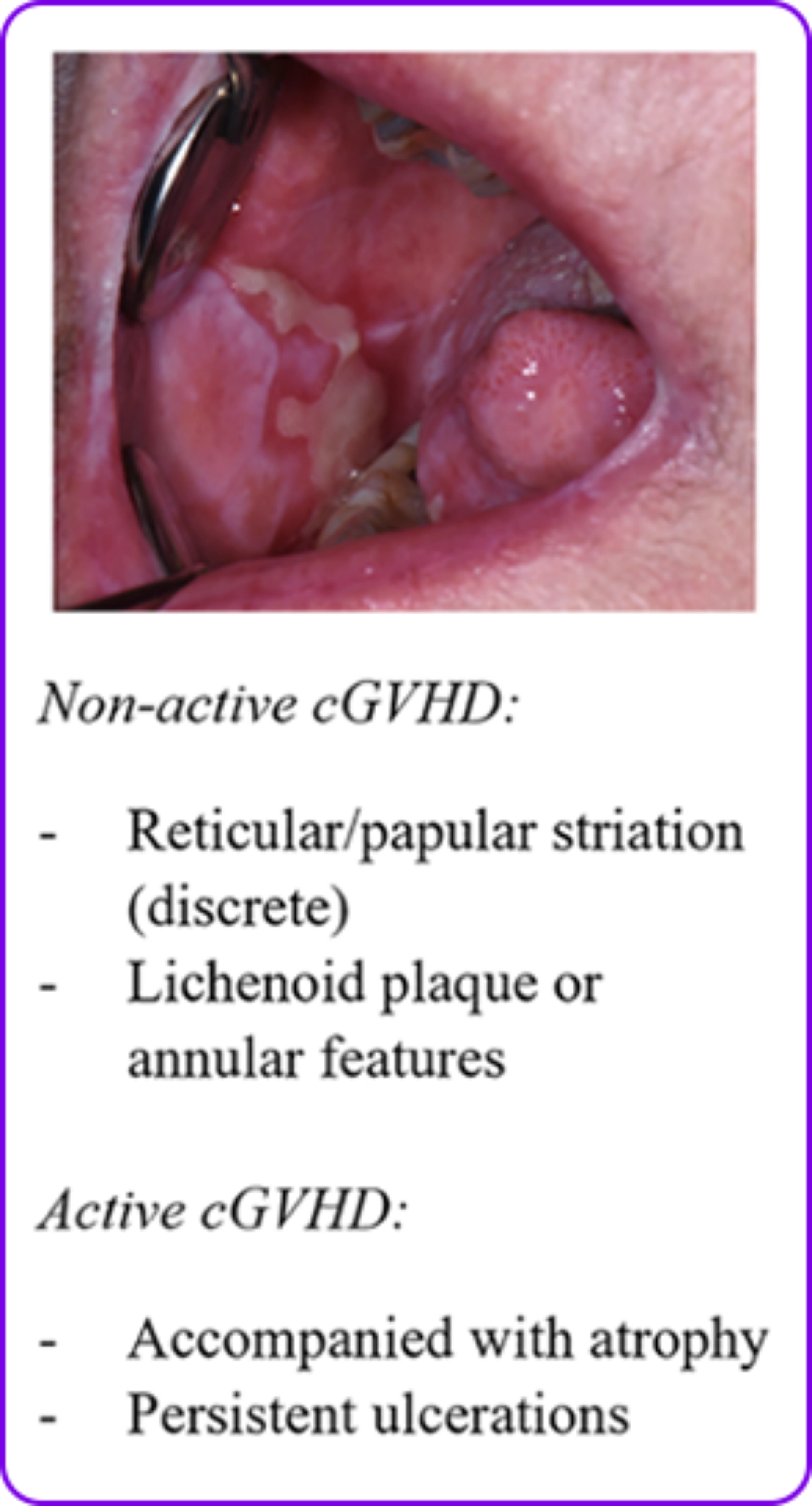
Lichen planus-like changes1
- Lichen planus-like changes that are characterized by hyperkeratotic white lines and lacy-appearing lesions on the oral mucosa
- Changes are typically observed on the buccal mucosa and tongue
Mouth: Distinctive
Ulcers2
- Xerostomia (dryness)2
- Mucosal atrophy2
- Ulcers2
- Mucoceles2
- Pseudomembranes
The mouth is a predominant site in pediatric patients with cGvHD3
- Lichenoid changes
- Hyperkeratotic plaques
- Circumoral restriction
- Impaired enamel
- Ageusia (loss of taste)
Scoring of cGvHD: Mouth4
Mouth4
| SCORE 0 | SCORE 1 | SCORE 2 | SCORE 3 | |
| MOUTH Lichen planus–like features present: by ophthalmologist: ☐ Yes ☐ No |
☐ No symptoms | ☐ Mild symptoms with disease signs but not limiting oral intake significantly |
☐ Moderate symptoms with disease signs with partial limitation of oral intake |
☐ Severe symptoms with disease signs on examination with major limitation of oral intake |
| ☐ Abnormality present but explained entirely by non-GVHD documented cause (specify): | ||||
cGvHD, chronic graft-versus-host disease.
- Margaix-Muñoz M et al. J Clin Exp Dent. 2015;7(1):e138-e145. doi:10.4317/jced.51975;
- Mawardi H et al. Oral Dis. 2019;25(4):931-948. doi:10.1111/odi.12936;
- Yanik G. Pediatric transplant: issues. Lecture presented at: The Pediatric Blood and Marrow Transplant Program; April 2022;
- Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001.
The figures are included from the article Rodrigues S K et al. Am J Clin Dermatol. 2018 Feb;19(1):33-50; which is under the copyright license of CC-BY-NC that permits the non-commercial use.
Management
Treatment of oral cGvHD1
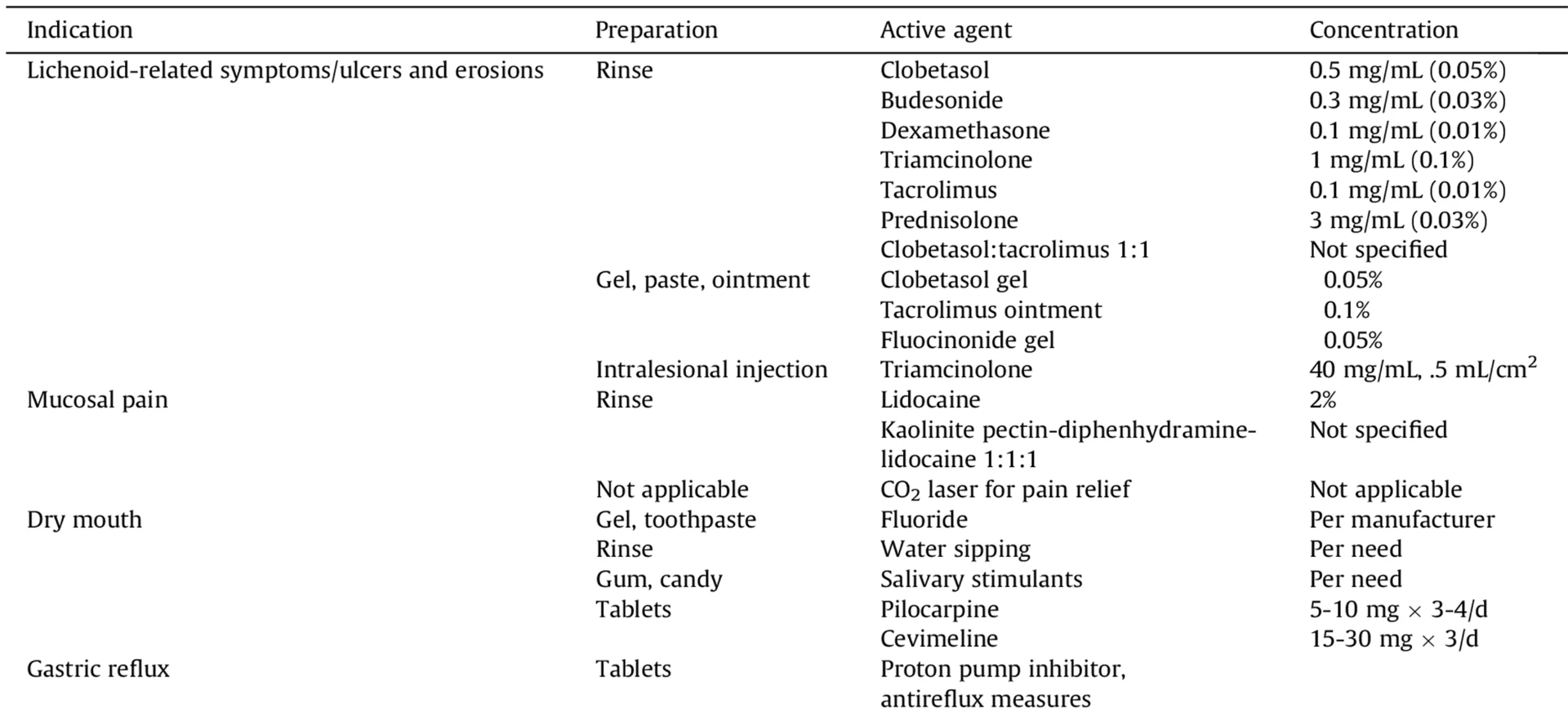

- Behavioural: Children often don’t communicate symptoms of impaired speech, oral dryness or sensitivities, taste alteration, and dysphagia; reduced oral intake and increased drinking during eating or at night may be presenting symptoms. Specific approaches to help parents assist children with oral topical therapies may improve compliance.
- Secondary infections with viruses (especially herpes simplex) and yeasts are common; therefore, using a local antifungal preparation in combination with the steroid rinse is recommended.
- Developmental: Fibrosis and limited mouth opening may contribute to the disruption of craniofacial growth.
- Pharmacologic considerations: While oral mucosal cGvHD generally responds well to topical steroids or calcineurin inhibitors, these agents may produce clinically relevant systemic drug levels in infants and small children. Viscous lidocaine may reduce the gag reflex, compromise swallowing and should be used with caution. Sialagogue use has been limited in children and dosing is not established.
- Orthodontic: Fixed braces should only be used after healing of acute inflammation. Specific guidelines for optimal force and pace of orthodontic management in cGvHD remain undefined.
cGvHD, chronic graft-versus-host disease.
- Carpenter PA et al. Biol Blood Marrow Transplant. 2015;21(7):1167-87
Types of BOS: Lungs
- BOS is the lung manifestation of cGvHD that is clinically characterized by the development of fixed new-onset airflow obstruction and pathologically characterized by progressive fibrosis targeting the terminal bronchioles.1
- BOS clinically presents as obstructive lung disease detected as a decline in FEV1. BOS is the predominant phenotype of chronic lung allograft dysfunction, which remains a major cause of morbidity and mortality.2
- One challenge in diagnosing BOS in pediatric patients is the inability to accurately measure FEV1 in children aged >8 years.3
- BOS can occur secondary to cGvHD or secondary to lung transplantation.4 The following slides will discuss BOS secondary to cGvHD.
Proliferative BOS5,6
|
Air-space opacification and intraluminal exudates; much more common and likely to respond to therapy. |
Constrictive BOS3,5
|
Alterations in the walls of the bronchioles ranging from inflammation to fibrosis and, ultimately, to complete obliteration of the lumen.5 One challenge in pediatric patients is recognizing this type of BOS.3 |
BOS is a progressive disease that is associated with high morbidity and mortality rates.4
- Among 2859 patients with cGvHD, the incidence of BOS was 6% at 1 year and 7% at 3 years after transplantation.7
- Patients affected by BOS have a 2-year survival rate of 44% and a 5-year survival rate of 13%.8
Overview of BIOS in cGvHD
| Epidemiology1,8 |
Risk factors associated with the development of BOS after allo-HCT include8
Risk factors found to be significantly associated with cGvHD include1
|
cGvHD, chronic graft-versus-host disease; BOS, bronchiolitis obliterans syndrome; FEV1, forced expiratory volume at 1 second. alloHCT, allogeneic hematopoietic cell transplant; FEV1, forced expiratory volume at 1 second.
- Au BKC et al. Biol Blood Marrow Transplant. 2011;17(7):1072-1078. doi:10.1016/j.bbmt.2010.11.018;
- Pilewski J. Chronic lung allograft dysfunction: bronchiolitis obliterans syndrome. UpToDate. Accessed April 11, 2022. https://www.uptodate.com/contents/chronic-lung-allograft-dysfunction-bronchiolitis-obliterans-syndrome
- . Yanik G. Pediatric transplant: issues. Lecture presented at: The Pediatric Blood and Marrow Transplant Program; April 2022;
- Williams KM et al. Transplant Cell Ther. 2022;S2666-6367(22)00049-5. doi:10.1016/j.jtct.2022.01.021;
- Smith KJ, Fan LL. Thorax. 2006;61(6):462-463. doi:10.1136/thx.2005.052670;
- Jamkhana Z. Bronchiolitis obliterans. Cancer Therapy Advisor. Published January 17, 2019. Accessed April 11, 2022. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/hospital-medicine/bronchiolitis-obliterans/;
- Dudek AZ et al. Biol Blood Marrow Transplant. 2003;9(10):657-666. doi:10.1016/S1083-8791(03)00242-8;
- Chien JW et al. Biol Blood Marrow Transplant. 2010;16(1 suppl):S106-S114. doi:10.1016/j.bbmt.2009.11.002;
Diagnosis
Characteristics of cGvHD: Lungs
Lungs: Diagnostic – Partial obstruction of bronchioles in BOS1
- Bronchiolitis obliterans2
- BOS2
- Characterized by peribronchiolar fibrosis, intraluminal fibrous obliteration and circumferential narrowing of the terminal small airways
- Clinical presentation shows airflow obstruction.3
Lungs: Distinctive2
- Air trapping and bronchiectasis
Lungs: Other2
- Cryptogenic organizing pneumonia
- Restrictive lung disease
Complete obstruction of bronchioles in BOS1
Scoring of cGvHD: Lungs4
Lungs4
| LUNGS** Symptom Score |
☐No symptoms |
☐ Mild symptoms
|
☐ Moderate symptoms (shortness of breath after walking on flat ground) |
☐ Severe symptoms (shortness of breath at rest; requiring O2) |
|
| Lung Score | ☐ FEV1 ≥ 80% | ☐ FEV1 60–79% | ☐ FEV1 40–59% | ☐ FEV1 ≤ 39% | |
|
Pulmonary function tests ☐ Not performed |
|||||
**Lung scoring should be performed using both the symptoms and FEV1 scores whenever possible. FEV1 should be used in the final lung scoring where there is discrepancy between symptoms and FEV1 scores.
cGvHD, chronic graft-versus-host disease; FEV1, forced expiratory volume at 1 second.
- Dudek AZ et al. Biol Blood Marrow Transplant. 2003;9(10):657-666. doi:10.1016/S1083-8791(03)00242-8;
- Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001;
- arrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001; 3. Bergeron A, Cheng G-S. Clin Chest Med. 2017;38(4):607-621. doi:10.1016/j.ccm/2017.07.003;
- Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001
Management
BOS secondary to cGvHD: Lungs
| Patient path to therapy1,2,3 |
Diagnosis1,2,3
General guidelines for treatment1,3
|
| Current treatment options1,4 |
|

- Formal spirometry, lung volumes, and diffusing capacity are not measurable reliably in children <7 years, but negative plethysmography is an option. The PFT screening can be tried beginning at 6 years of age, but results should take into consideration the child’s compliance with PFT instructions and proper technique, so it requires discussion with a pulmonologist.
- Actual measured PFT values must be carefully considered in pediatric patients because predicted normal values vary with age, weight, and height. Therefore, per cent predicted values might spuriously show a serial decrease over time without a substantive decline in absolute values.
- Recipients of total body or chest wall irradiation may not have proportional chest wall growth, and this is particularly relevant for growing children. A drop in the percent-predicted value may reflect poor lung growth rather than a physiologic drop in lung function.
BOS, bronchiolitis obliterans syndrome; cGvHD, chronic graft-versus-host disease; HCT, hematopoietic stem cell transplant; PFT, Pulmonary function test.
- Flowers MED, Martin PJ. Blood. 2015;125(4):606-615. doi:10.1182/blood-2014-08-551994;
- Dudek AZ et al. Biol Blood Marrow Transplant. 2003;9(10):657-666. doi:10.1016/S1083-8791(03)00242-8;
- Williams KM. Blood. 2017 Jan 26;129(4):448-455. doi: 10.1182/blood-2016-08-693507;
- Au BKC et al. Biol Blood Marrow Transplant. 2011;17(7):1072-1078. doi:10.1016/j.bbmt.2010.11.018;
- Carpenter PA et al. Biol Blood Marrow Transplant. 2015;21(7):1167-87
How GvHD affects Liver
Hepatic GvHD presents with abnormal liver function tests, along with elevated levels of bilirubin and alkaline phosphatase in the serum.
Donor lymphocytes target bile duct epithelial cells, leading to endotheliitis, pericholangitis, and the destruction of bile ducts through apoptosis.
Liver GvHD causing bile duct involvement and resulting in severe hyperbilirubinemia is less common, there is a rising incidence of hepatitis-like cGvHD as another, albeit less harmful, liver complication.
cGvHD, chronic graft-versus-host disease.
1. Ghimire S et al. Front Immunol. 2017. 10.3389/fimmu.2017.00079.
Diagnosis
Characteristics of cGvHD: Liver1
Liver: Common for both aGvHD and cGvHD
- Increased LFTs (i.e., total bilirubin, alkaline phosphatase [AP], alanine transaminase [ALT])
- Total bilirubin, ALP or ALT >2 x upper normal liimt
Scoring of cGvHD: liver2
liver2
| SCORE 0 | SCORE 1 | SCORE 2 | SCORE 3 | |
| LIVER |
☐ Normal total bilirubin and ALT or AP < 3 × ULN |
☐ Normal total bilirubin with ALT ≥ 3 to 5 × ULN or AP ≥ 3 × UL |
☐ Elevated total bilirubin but ≤3 mg/dL or ALT > 5 ULN |
☐ Elevated total bilirubin > 3 mg/dL |
| ☐ Abnormality present but explained entirely by non-GVHD documented cause (specify): | ||||
cGvHD, chronic graft-versus-host disease; GI, gastrointestinal; LFTs, liver function tests; ALT, alanine aminotransferase; AP, alkaline phosphatase; ULN, normal upper limit.
- Zeiser R, Blazar BR. N Engl J Med. 2017;377(26):2565-2579.doi:10.1056/NEJMra1703472.
- Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001.
Management
Treatment of Liver1
- Surveillance for infection (viral, bacterial, fungal, parasites)
- Rule out other potential etiologies
- Dietary modification for carbohydrate, fat malabsorption
- Pancreatic enzyme replacement for insufficiency
- Ursodeoxycholic acid if cholestasis
- Bile acid binding resins if bile salt malabsorption (e.g., cholestyramine)
- Lactase tablets or lactase-containing dairy products
- Topical glucocorticoid formulations for late acute GVHD
- Limitation of ethanol intake
- Avoidance of hepatotoxins

- Children with cGvHD may have varied GI complaints consisting of nausea, anorexia, abdominal pain, weight loss, cramping or diarrhea.
- Weight loss and malnutrition (as reflected by a decrease in BMI) are clinically significant issues in children with multisystem cGvHD and are likely systemic manifestations of the disease.
- Maintaining adequate nutrition is essential and careful evaluation of growth and head circumference in infants is required.
BMI, body mass index; cGvHD, chronic graft-versus-host disease; GI, gastrointestinal.
- Carpenter PA et al. Biol Blood Marrow Transplant. 2015;21(7):1167-87.
How GvHD affects skin
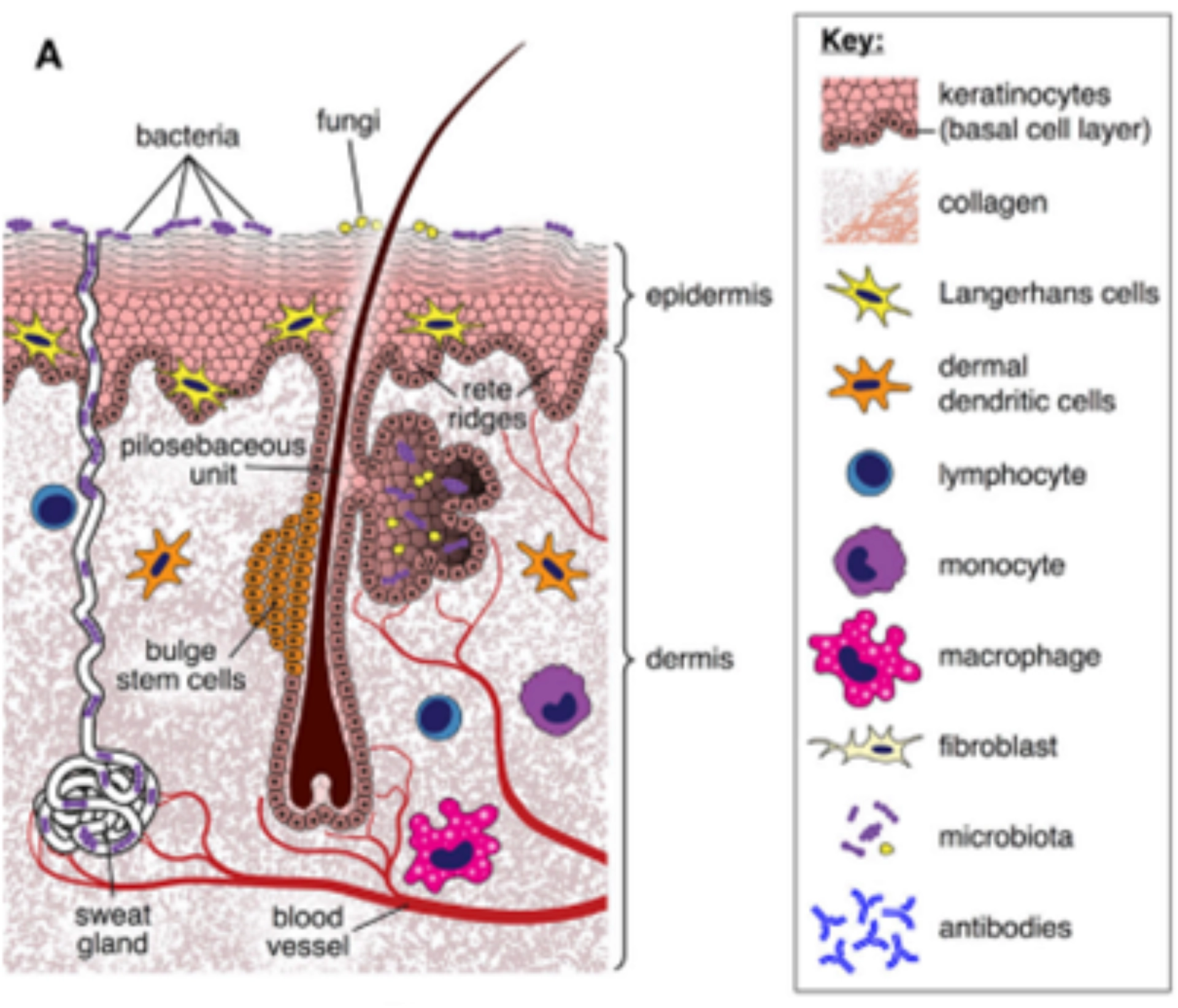
Figure 1: Normal Skin
cGvHD involves complex mechanisms such as thymic injury, disordered T cell selection, loss of regulatory cells, disrupted B cell homeostasis, and abnormal tissue repair leading to fibrosis.1
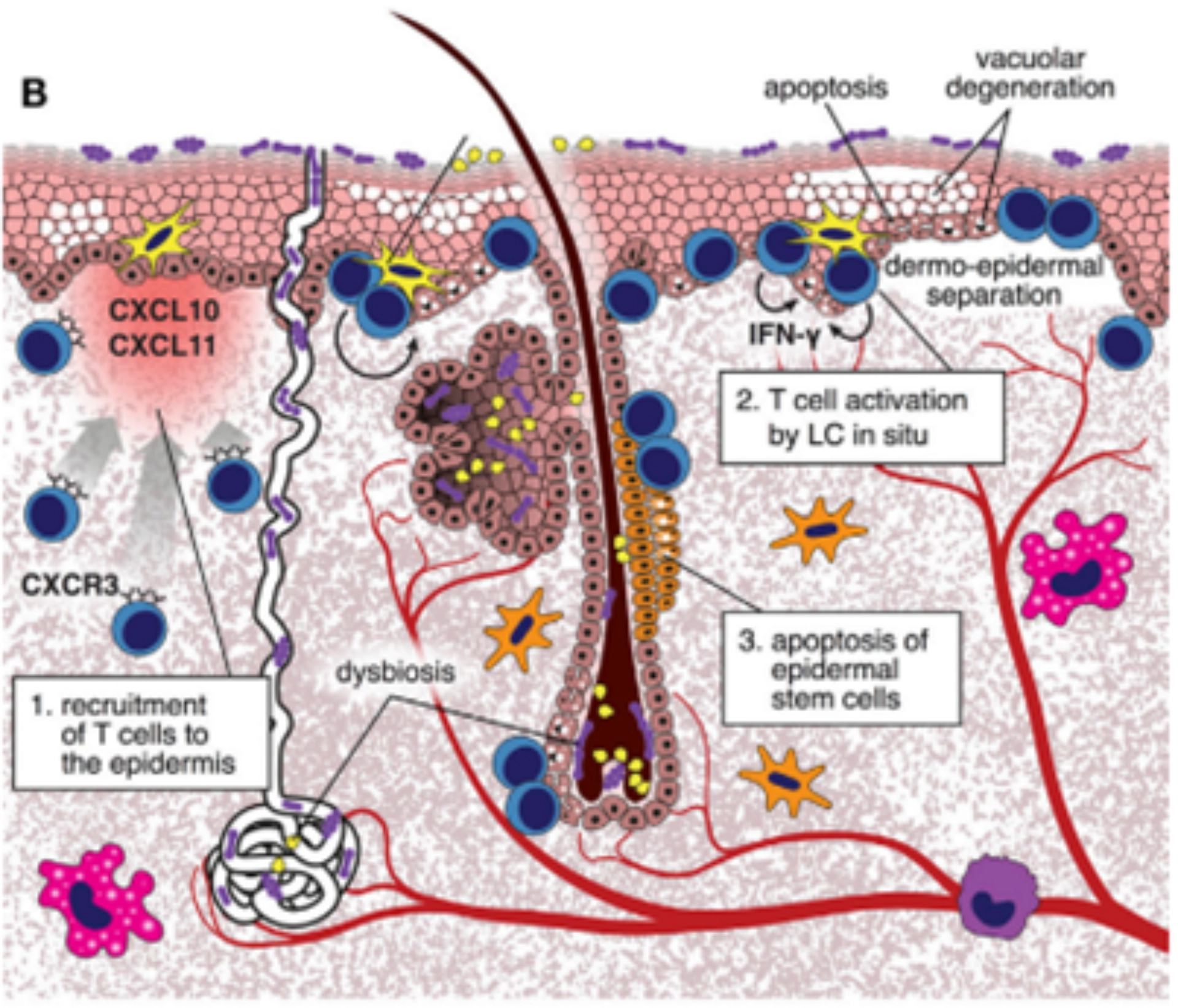
Figure 2: aGvHD process
During aGvHD, tissue injury triggers the production of IFN-γ inducible chemokines, attracting CXCR3+ alloreactive T cells to the skin. These T cells interact with host Langerhans cells, becoming pathogenic and producing IFN-γ. They then destroy Lgr5+ epidermal stem cells, impairing skin repair and causing cell degeneration, separation, and necrosis. Additionally, changes in the local microbiome due to treatment can exacerbate skin inflammation.
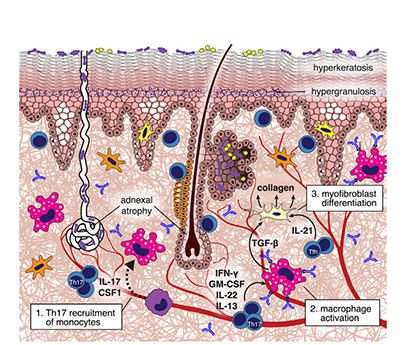
Figure 3: cGvHD process
During chronic GvHD, donor T cell-derived IL-17 recruits Ly6Clow monocytes to the skin, which become macrophages. These macrophages produce TGF-β, leading to fibroblast differentiation into myofibroblasts that deposit collagen. Allo- or auto-antibodies further polarize macrophages to produce more TGF-β, enhancing collagen production. IL-21 from follicular helper-like cells also affects fibroblast differentiation, resulting in thickened and homogenized collagen bundles in the dermis.
aGvHD, acute Graft-Versus-Host Disease; CXCR3, C-X-C Motif Chemokine Receptor 3; cGvHD, chronic Graft-Versus-Host Disease; IFN-γ, Interferon Gamma; IL-17, Interleukin 17; IL-21, Interleukin 21; Lgr5, Leucine-rich repeat-containing G-protein coupled receptor 5; Ly6Clow, Low Ly6C Expression; TGF-β, Transforming Growth Factor Beta.
- Pedro Santos e S et al. Front Immunol. 2018;9:00963.
Diagnosis
Characteristics of cGvHD: Skin
Skin: Diagnostic Poikiloderma1
- Poikiloderma (i.e., atrophy, pigmentary changes, telangiectasia)2
- Lichen planus-like eruption (i.e., erythematous flat-topped papules/plaques with/without a shiny appearance)2
- Deep sclerotic features (i.e., smooth, waxy, thickened/tight skin due to deep and diffuse sclerosis over a large area limiting joint mobility)2
- Morphea-like features (i.e., localized patchy areas of moveable smooth/shiny skin with leather-like consistency that often has dyspigmentation)2
Papulosquamous lesions3
- Depigmentation (vitiligo)
- Papulosquamous lesions
- Sclerosis
- Morphea-like
- Lichenslcerosis-like
Skin: Other Hyperpigmentation4
- Sweat impairment and intolerence to temperature change due to loss of sweat glands
- Keratosis pilaris
- Ichthyosis
- Hypopigmentation
- Hyperpigmentation
Scoring of cGvHD: Skin, nails, and hair5
Skin5
| SCORE 0 | SCORE 1 | SCORE 2 | SCORE 3 | |
|
PERFORMANCE SCORE: KPS ECOG LPS |
☐ Asymptomatic and fully active (ECOG 0; KPS or LPS 100%) |
☐ Symptomatic, fully ambulatory, restricted only in physically strenuous activity (ECOG 1, KPS or LPS 80–90%) |
☐ Symptomatic, ambulatory, capable of self-care, >50% of waking hours out of bed (ECOG 2, KPS or LPS 60–70%) |
☐ Symptomatic, limited self-care, >50% of waking hours in bed (ECOG 3–4, KPS or LPS <60%) |
|
SKIN: SCORE % BSA GVHD features to be scored by BSA: Check all that apply: ☐Maculopapular rash/erythema ☐ Lichen planus–like features ☐Sclerotic features ☐Papulosquamous lesions or ichthyosis ☐Keratosis pilaris–like GVHD
|
☐ No BSA involved | ☐ 1 - 18% BSA | ☐ 19-50% BSA | ☐ >50% BSA |
|
SKIN FEATURES: SCORE : |
☐ No sclerotic features |
☐ Superficial sclerotic features "not hidebound" (able to pinch) |
Check all that apply
☐ Deep sclerotic features ☐ "Hidebound" (unable to pinch) ☐ impaired mobility ☐ Ulceration |
|
|
Other skin GVHD features (NOT scored by BSA) Check all that apply: ☐Hyperpigmentation ☐Hypopigmentation ☐Poikiloderma ☐Severe or generalized pruritus ☐ Hair involvement ☐ Nail involvement ☐ Abnormality present but explained entirely by non-GVHD documented cause (specify): |
||||
†Skin scoring should use both percentage of BSA involved by disease signs and the cutaneous features scales. When a discrepancy exists between the percentage of total body surface (BSA) score and the skin feature score, OR if superficial sclerotic features are present (Score 2), but there is impaired mobility or ulceration (Score 3), the higher level should be used for the final skin scoring.
cGvHD, chronic graft-versus-host disease; BSA, body surface area; ECOG, Eastern Cooperative Oncology Group; KPS, Karnofsky Performance Status; LPS, Lansky Performance Status.
- Hymes SR, et al. Biol Blood Marrow Transplant. 2006. https://doi.org/10.1016/j.bbmt.2006.08.043;12(11):1101-13;
- Pavletic SZ et al. Bone Marrow Transplant. 2006;38(10):645-651. doi:10.1038/sj.bmt.1705490;
- Jang S et al. Ann Dermatol. 2016;28(1):90-93. doi:10.5021/ad.2016.28.1.90;
- Gray AN, et al. JAAD Case Rep. 2023;36:82-88. doi: 10.1016/j.jdcr.2023.03.023;
- Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001.
The figures are included from the article Rodrigues S K et al. Am J Clin Dermatol. 2018 Feb;19(1):33-50; which is under the copyright license of CC-NC that permits the non-commercial use
Management
Treatment of skin GvHD1
Preventive measures
Photoprotection: UVA and UVB blockade including:
- Avoidance of sun exposure (especially between 10:00 am and 4:00 pm)
- Use of sunscreens (> SPF 20 with broad-spectrum UVA and UVB protection)
- Protective clothing
- Avoidance of photosensitizing agents
Treatment
Intact skin
- Symptomatic treatment with emollients and antipruritic agents
- Topical corticosteroids
- Light therapy (PUVA, UVA1, UVB, narrow-band UVB)
- Topical calcineurin inhibitors (pimecrolimus, tacrolimus)
Sclerotic manifestations with joint stiffness or contractures
- Deep muscle/fascial massage (Heller works) to improve range of motion
- Stretching exercises to improve range of motion
Erosions and ulcerations
- Topical or oral antimicrobials
- Wound dressings and debridement
- Control of edema

- Systemic side effects of topical steroids and calcineurin inhibitors may occur more frequently in young children because of the larger skin surface area to body weight ratio.
- Less potent steroids [Hydrocortisone 1-2.5%] should be used for thinner areas [face, neck, axillae, and groin]and higher potency steroids [Betamethasone 0.1%] are recommended for rest of the areas. For sclerotic form of cGvHD clobetasol 0.05% can be used. It should be applied twice daily.
- Topical tacrolimus is commonly used as an adjunct treatment in practice and applied twice a day with at least 1 hour gap from steroid application. [0.03% strength for face and genitalia and 0.1% strength for rest of the body]. It should be considered before increasing the dose of systemic immune suppressants or to reduce exposure to steroids.
- Topical steroids under occlusive dressings are not recommended.
- Avoid urea or menthol containing products in young children because they may cause skin irritation.
- Skin care include topical moisturizers, antipruritic agents, and strict photoprotection
cGvHD, chronic graft-versus-host disease; GvHD, graft-versus-host disease; PUVA, Psoralen plus ultraviolet A; SPF, Sun Protection Factor; UVA, Ultraviolet A; UVB, Ultraviolet B.
Carpenter PA et al. Biol Blood Marrow Transplant. 2015;21(7):1167-87
How GvHD affects GI tract1
The base of intestinal crypts, housing epithelial stem cells and Paneth cells, is a key target for GvHD due to its role in epithelium regeneration. Loss of Paneth cells at GI GvHD onset suggests their sensitivity to the condition.1
Histological features such as single cell apoptosis, patchy ulcerations, apoptotic bodies in crypt bases, loss of surface epithelium can be reported.
This mechanism results in secretory diarrhea, severe abdominal pain, vomiting, and anorexia.
GVHD development in the gut is associated with a disturbed microbiome. In cGvHD decreased microbial diversity with reduced Faecalibacterium and increased Akkermansia and Streptococcus species are reported, whereas high abundance of Lachnoclostridium is protective.2
cGvHD, chronic graft-versus-host disease; GI, gastrointestinal.
- Ghimire S et al. Front Immunol. 2017. 10.3389/fimmu.2017.00079.
- Gail LM et al. Front Immunol. 2023:14:1199422.
Diagnosis
Characteristics of cGvHD: GI tract1
GI tract: Common for both aGvHD and cGvHD
- Anorexia
- Nausea
- Vomiting
- Diarrhea
- Weight loss
GI tract: Diagnostic
- Sclerotic mucosa of the esophagus1
- Esophageal webs, strictures or concentric rings documented by endoscopy or barium contrast radiograph
GI tract: Other
- Exocrine pancreatic insufficiency
Scoring of cGvHD: GI tract2
GI tract2
|
SCORE 0
|
SCORE 1 | SCORE 2 | SCORE 3 | |
|---|---|---|---|---|
|
GI Tract ☐ Esophageal web/ proximal stricture or ring |
☐ No symptoms |
☐ Symptoms without significant weight loss* (<5%) |
☐ Symptoms associated with mild to moderate weight loss* (5–15%) OR moderate diarrhea without significant interference with daily living |
☐ Symptoms associated with significant weight loss* >15%, requires nutritional supplement for most calorie needs OR esophageal dilation OR severe diarrhea with significant interference with daily living |
| ☐ Abnormality present but explained entirely by non-GVHD documented cause (specify): | ||||
*Weight loss within 3 months.
cGvHD, chronic graft-versus-host disease; GI, gastrointestinal.
- Zeiser R, Blazar BR. N Engl J Med. 2017;377(26):2565-2579.doi:10.1056/NEJMra1703472;
- Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001
Management
Treatment of GI tract1
- Surveillance for infection (viral, bacterial, fungal, parasites)
- Rule out other potential etiologies
- Gastroesophageal reflux management
- Esophageal dilation for webs or stricture
- Dietary modification for carbohydrate, fat malabsorption
- Pancreatic enzyme replacement for insufficiency
- Ursodeoxycholic acid if cholestasis
- Bile acid binding resins if bile salt malabsorption (e.g., cholestyramine)
- Lactase tablets or lactase-containing dairy products
- Topical glucocorticoid formulations for late acute GVHD
- Limitation of ethanol intake

- Children with cGvHD may have varied GI complaints consisting of nausea, anorexia, abdominal pain, weight loss, cramping or diarrhea.
- Weight loss and malnutrition (as reflected by a decrease in BMI) are clinically significant issues in children with multisystem cGvHD and are likely systemic manifestations of the disease.
- Maintaining adequate nutrition is essential and careful evaluation of growth and head circumference in infants is required.
GvHD, graft-versus-host disease; BMI, body mass index; cGvHD, chronic graft-versus-host disease; GI, gastrointestinal.
1. Carpenter PA et al. Biol Blood Marrow Transplant. 2015;21(7):1167-87.
How cGvHD affects Genitalia1
- Genital cGvHD presents at a median of 7–10 months after HCT, but signs and symptoms can also develop late, over a year post-HCT.
- GVHD of the genital tract is often associated with oral cGvHD. Genital examination is recommended, even in asymptomatic patients, especially if signs of cGvHD are present in the mouth.
- Symptoms of genital cGvHD in women are due to estrogen deficiency and include vulvovaginal dryness, pruritis, burning, pain, dysuria, dyspareunia and at times, bleeding. Patients who are sexually active may experience pain and post-coital bleeding and thus report symptoms earlier.
- Acute and chronic inflammation within the subepithelial stroma and epithelium, with vacuolar degeneration of the basal layer, apoptotic keratinocytes and superficial perivascular lymphohistiocytic infiltrates.
- Typical signs such as erythema of the vulva, tenderness of vestibular gland openings, mucosal erosions or fissures, lace-like leukokeratosis or vulvar architecture changes such as labial resorption or clitoral hood agglutination are seen.
- Male genital lesions post-transplant include lichenoid, lichen sclerosis, phimosis, and contracture secondary to Peyronie’s disease, which may lead to penile deformity, pain, and erectile dysfunction.
cGvHD, chronic graft-versus-host disease; GvHD, graft-versus-host disease; HCT, hematopoietic cell transplantation
1. Hamilton BK et al. Bone Marrow Transplant. 2017;52(6):803-810
Diagnosis
Characteristics of cGvHD: Genitalia
Genitalia: Diagnostic
Labial agglutination1-3
- Lichen planus-like and lichen sclerosis-like features
- Females: vaginal scarring or clitoral/labial agglutination
- Males: phimosis and urethral scarring or stenosis
Genitalia: Distinctive
- Erosion
- Fissure
- Ulcers
Scoring of cGvHD: Genitalia4
Genital tract4
| SCORE 0 | SCORE 1 | SCORE 2 | SCORE 3 | |
|
GENITAL TRACT Currently sexually active |
☐ No signs |
☐ Mild signs and on exam |
☐ Moderate signs and may have symptoms with discomfort on exam |
☐ Severe signs with or without symptoms |
| ☐ Abnormality present but explained entirely by non-GVHD documented cause (specify): | ||||
‡To be completed by specialist or trained medical providers.
cGvHD, chronic graft-versus-host disease; KCS, keratoconjunctivitis sicca; ADL, activities of daily living.
- Nassiri N et al. J Ophthalmic Vis Res. 2013;8(4)::351-358;
- Screening eyes for chronic GvHD. BetheMatchClinical.org. Accessed April 11, 2022. https://bethematchclinical.org/post-transplant-care/chronic-gvhd/eyes/;
- Smith Knutsson E et al. Acta Obstet Gynecol Scand. 2018;97(9):1122-1129. doi:10.1111/aogs.13366
- Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001.
The figures are included from the article Nair S et al. Indian J Ophthalmol. 2021 May;69(5):1038-1050; which is under the copyright license of CC-BY-NC that permits the non-commercial use
Management
Treatment of genitalia1
- Early gynecology consultation
- Water-based or silicone lubricants
- Topical estrogens
- Topical corticosteroids or calcineurin inhibitors
- Dilators or vibrators
- Surgery for extensive synechiae or obliteration
- Surveillance for estrogen deficiency, infection (HSV, HPV, yeast, bacteria) and malignancy
- Avoid glycerin, paraben, fragrance, and other additive products

- Examine for evidence of phimosis and glans dryness/ulceration periodically post-HSCT. Pre-BMT exam should document if patient has preexisting phimosis or not.
- Vulvar or vaginal GvHD needs to be considered as soon as physical development has progressed beyond thelarche and into pubarche.
- Vulvovaginal GvHD has been observed infrequently in prepubertal girls.
- Evaluation by an adolescent gynecologic practitioner is recommended when a diagnosis of vulvovaginal GvHD is being considered.
HPV, Human Papillomavirus; HSV, Herpes Simplex Virus; BMT, Bone marrow transplant; GvHD, graft-versus-host disease; HSCT, hematopoietic stem-cell transplantation.
1. Carpenter PA et al. Biol Blood Marrow Transplant. 2015;21(7):1167-87.
How cGvHD affects Fascia and joints1
- Joint and fascial involvement is relatively common and can cause significant functional impairment
- Fasciitis due to inflammation of the fascia with an eosinophilic component may manifest as joint stiffness, erythema, edema, restricted range of motion, arthralgia, and rarely arthritis or synovitis
- Joint and fascial manifestations can be clinically detectable when inflammation and fibrosis arise in deep tissues (deep sclerosis/fasciitis) or skin overlying joints (superficial sclerosis)
- Widespread sclerosis may result in joint contractures and severe limitation of function, and common sites of involvement include the hands/wrists, shoulders, elbows, and ankles
- Calleja CH et al. Reumatol Clin (Engl Ed).2023;19(5):235-243.
Diagnosis
Characteristics of cGvHD: Fascia and joints
Fascia and joints: Diagnostic
Subcutaneous and fascial fibrosis1,2
- Fasciitis
- Joint stiffness or contractures secondary to fasciitis or sclerosis
Joint contractures2
Fascia and joints:
Distinctive polymyositis3
- Myositis or polymyositis
Other
- Edema
- Muscle cramps
- Arthralgia or arthritis
- Scoring of cGvHD: Fascia and joints4
Scoring of cGvHD: Fascia and joints4
Joints and Fascia4
| SCORE 0 | SCORE 1 | SCORE 2 | SCORE 3 | |
|
JOINTS AND FASCIA Elbow (1-7): Wrist/finger (1-7): Ankle (1-4): |
☐ No symptoms | ☐ Mild tightness of arms or legs normal or mild decreased range of motion (ROM) AND not affecting ADL |
☐ tightness of arms or legs OR joint contractures, erythema thought due to fasciitis, moderate decrease ROM AND mild to moderate limitation of ADL |
☐ Contractures WITH significant decrease of ROM AND significant limitation of ADL (unable to tie shoes, butoon shirts, dress self etc.) |
| ☐ Abnormality present but explained entirely by non-GVHD documented cause (specify): | ||||
cGvHD, chronic graft-versus-host disease.
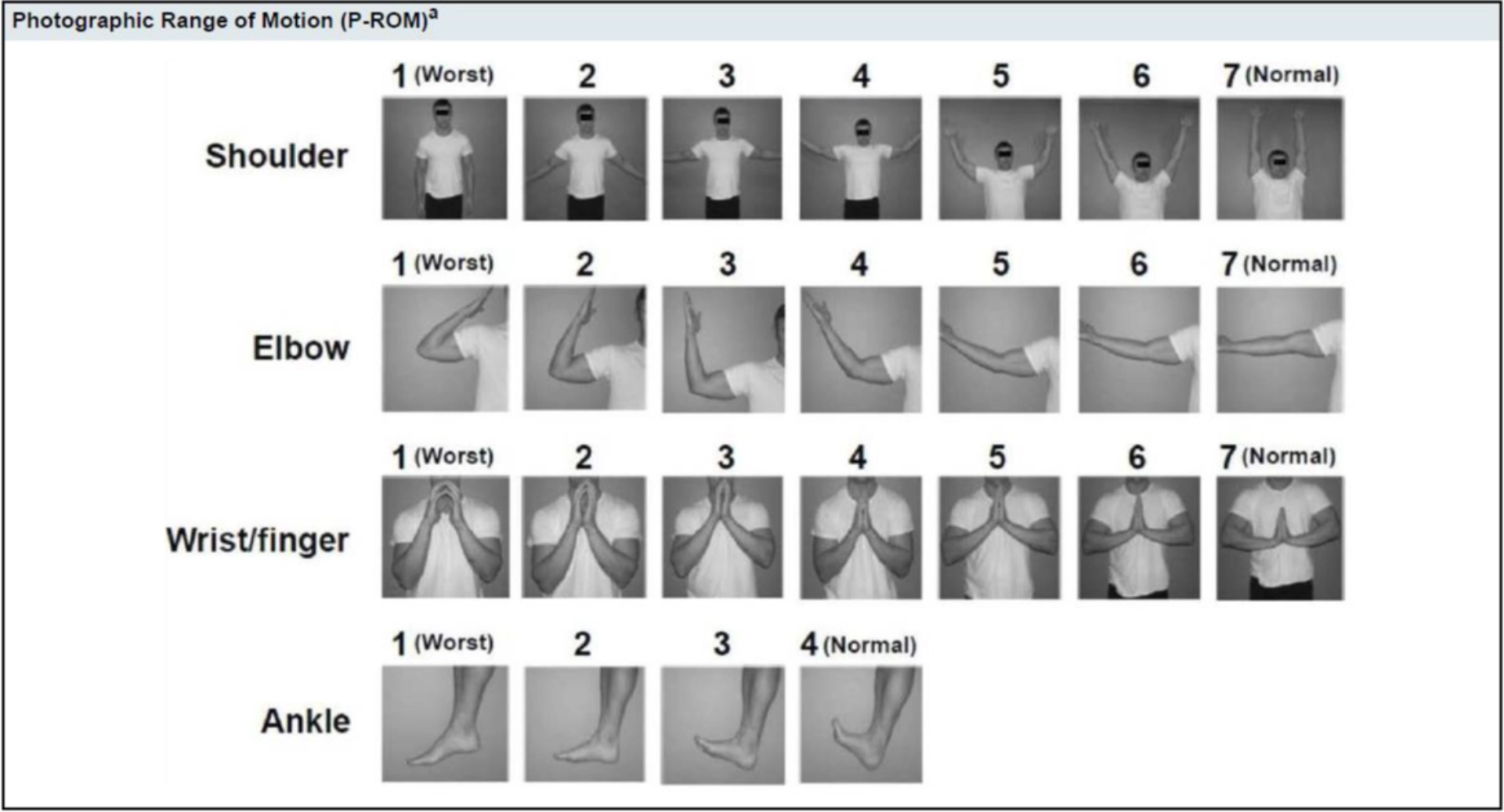
- Martires KJ et al.Blood. 2011;118(15):4250-4257. doi:10.1182/blood-2011-04-350249;
- Hidalgo Calleja C, et al. Adv Rheumatol. 202;62(1):33. doi: 10.1186/s42358-022-00262-3;
- Meng L et al. Clin Case Rep. 2018;6(9):1723–1726.doi:10.1002/ccr3.1709; 4. Jagasia MH et al. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. doi:10.1016/j.bbmt.2014.12.001
Treatment of joints
Polymyositis: Can be treated with the standard line therapies of steroids adjuvant with rituximab, only if there is a presence of B-cell infiltration.1
Systemic first line and second-line therapy: As per the NIH treatment guidelines it is essential to initiate the first line steroid treatment.2
Adjuvant treatment can be used based on the clinical presentation of the patient.2

- It is often challenging to examine the ROM of all joints in young kids. Particular attention should be paid to development and active trends at home, and parents should be instructed to observe this.
- Children should be monitored for complications of prolonged steroid therapy like avascular necrosis and osteoporosis.
NIH, National Institutes of Health; ROM, range of motion.
- Chinello M et al. Mediterr J Hematol Infect Dis. 2020;12(1):e2020002.
- DeFilipp Z et al. Transplant Cell Ther. 2021. 27(9):729-737.
- Carpenter PA et al. Biol Blood Marrow Transplant. 2015;21(7):1167-87