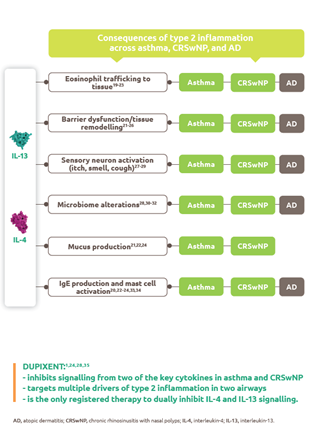
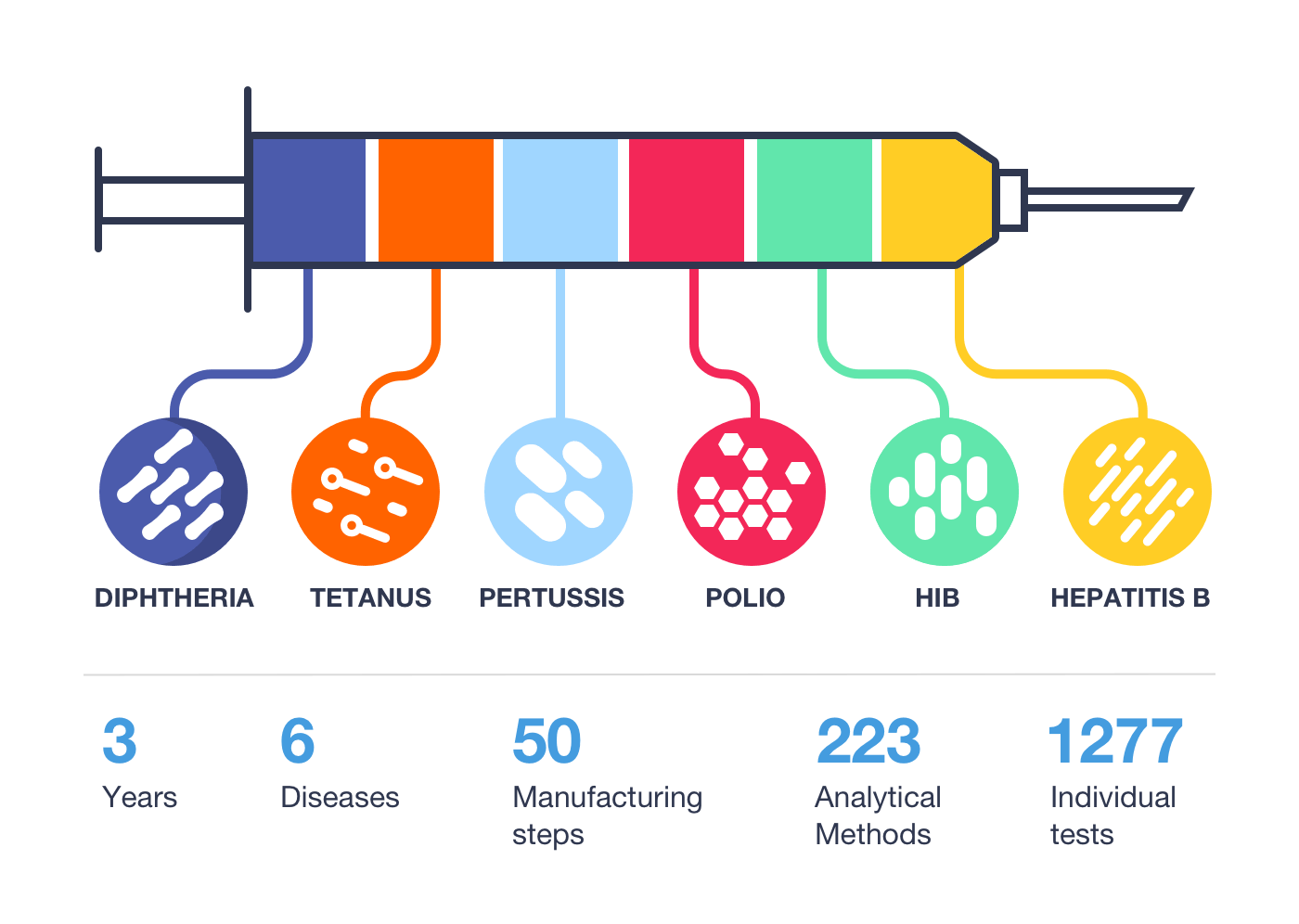
Vaccines: A Complex Manufacturing Process
Vaccines play an imperative role to help prevent potentially life-threatening infections such as meningitis, influenza, and polio.1 However, there is significant lead time between production and delivery to produce high-quality vaccines that meet public health needs. Production of vaccines delivered today may have commenced up to 36 months ago, and those in development now will be delivered in 2021-2022.2
For example, the hexavalent paediatric vaccine takes 3 years to manufacture with extensive manufacturing and testing procedures:
.jpg/jcr:content/ArticleImage_VaccineJourney%20(1).jpg)
.png)
How our vaccines are made in 7 steps:3-5
Step 1 - Production
Viruses or bacteria are grown in the lab. Temperature, pH, oxygen rate, sterility and homogeneity must be measured to ensure consistency. The slightest variation in the culture media may compromise production and cause delays. Depending on the vaccine, cell culture takes 2 days to 3 months. A small amount of original cell culture solution can be used to produce millions of vaccine doses.
Step 2 - Harvesting
The antigens produced from microorganisms are extracted. A small amount of original cell culture solution can be used to produce millions of vaccine doses.
Step 3 - Purification
Microorganisms are extracted from their environment, eliminating any trace of culture media. Impurities are removed and concentrated through physical and chemical processes.
Step 4 - Inactivation
The virus or bacteria are inactivated, eliminating its ability to cause disease. However, the ability to elicit a precautionary immune response from the body is retained.

Step 5 - Valence assembly
The active antigenic substances are combined in a single component.
_0.png/jcr:content/VaccineManufacturingProcess_Step6@2x%20(1)_0.png)
Step 6 - Formulation
A vaccine can include one or several valences to protect against one or multiple diseases in the same shot. However, each valence must remain fully effective, and not interfere with one another. Stabilisers or preservatives are added in extremely small quantities to help guarantee that the vaccine will remain stable, potent, and effective and sometimes an adjuvant is added to enhance the immune response.
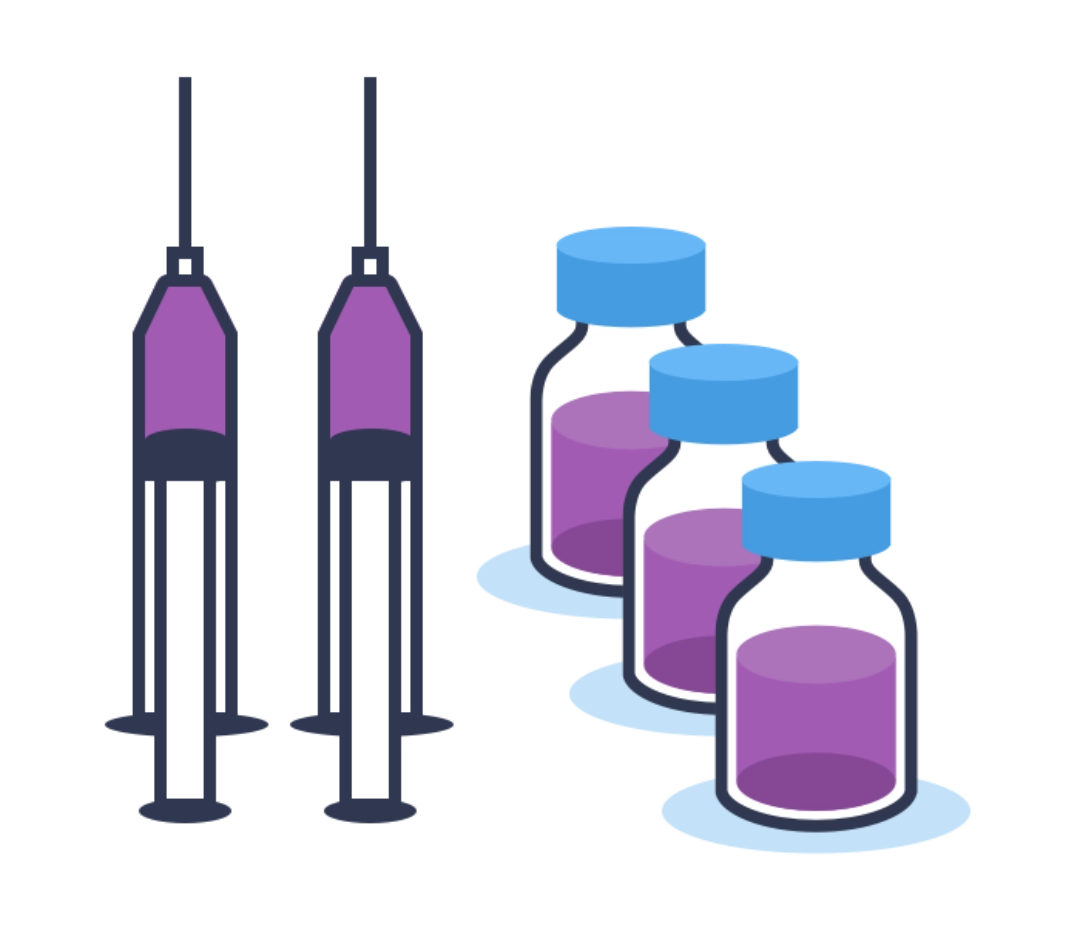
Step 7 - Filling
The vaccine in liquid or freeze-dried form, and diluents as needed, are filled into vials or syringes. The quality of the contents and the container are visually scrutinised by both the human eye and state-of-the-art digital surveillance technology.
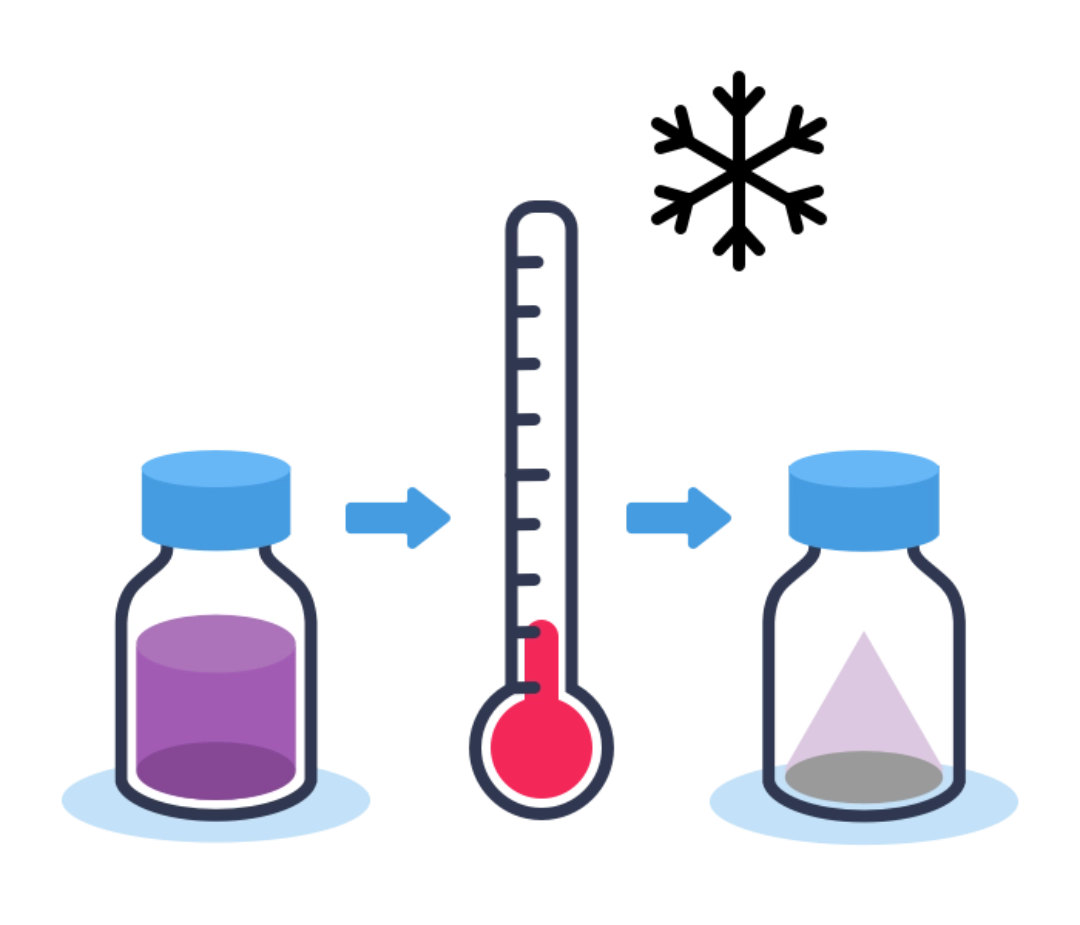
Step 8 - Freeze-drying
This step makes it possible to remove the water in a product by transforming it into a powder, which ensures better stability and conservation.

Step 9 - Packaging
The vaccine is labelled in accordance with regulatory requirements and packed, ready for shipping.

Step 10 - Batch release
Sanofi performs hundreds of laboratory tests on each and every batch of vaccine to confirm quality. In addition, samples are taken from each batch and sent to public health authorities for testing as well. Only after the vaccines have successfully passed both sets of tests can distribution begin. If a batch does not meet the quality criteria, it will be destroyed. Strict precautions are followed throughout the manufacturing process to ensure highest vaccine quality, including sterile working conditions (e.g. purified air), and continuous control along the production chain. Quality assurance confirms the product has been tested in accordance with the correct procedures. The national regulatory authority gives the final authorisation to release the product for distribution.
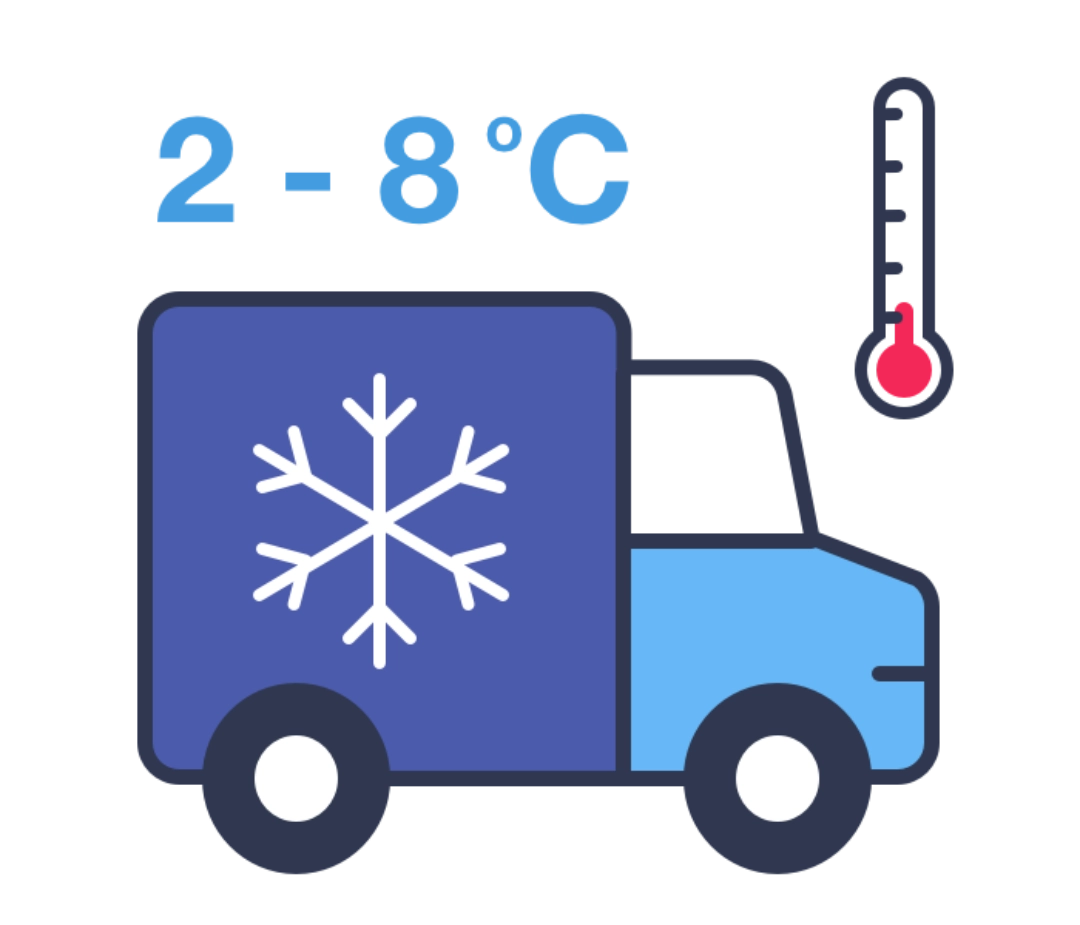
Step 11 - Transport
Throughout packaging, shipping and global distribution, vaccines must be stored between 2 and 8 °C. Various methods are used to maintain this temperature including refrigerated containers, isothermal packaging, and temperature tracking devices.
.png)
|
Want to learn more about the vaccine journey? |
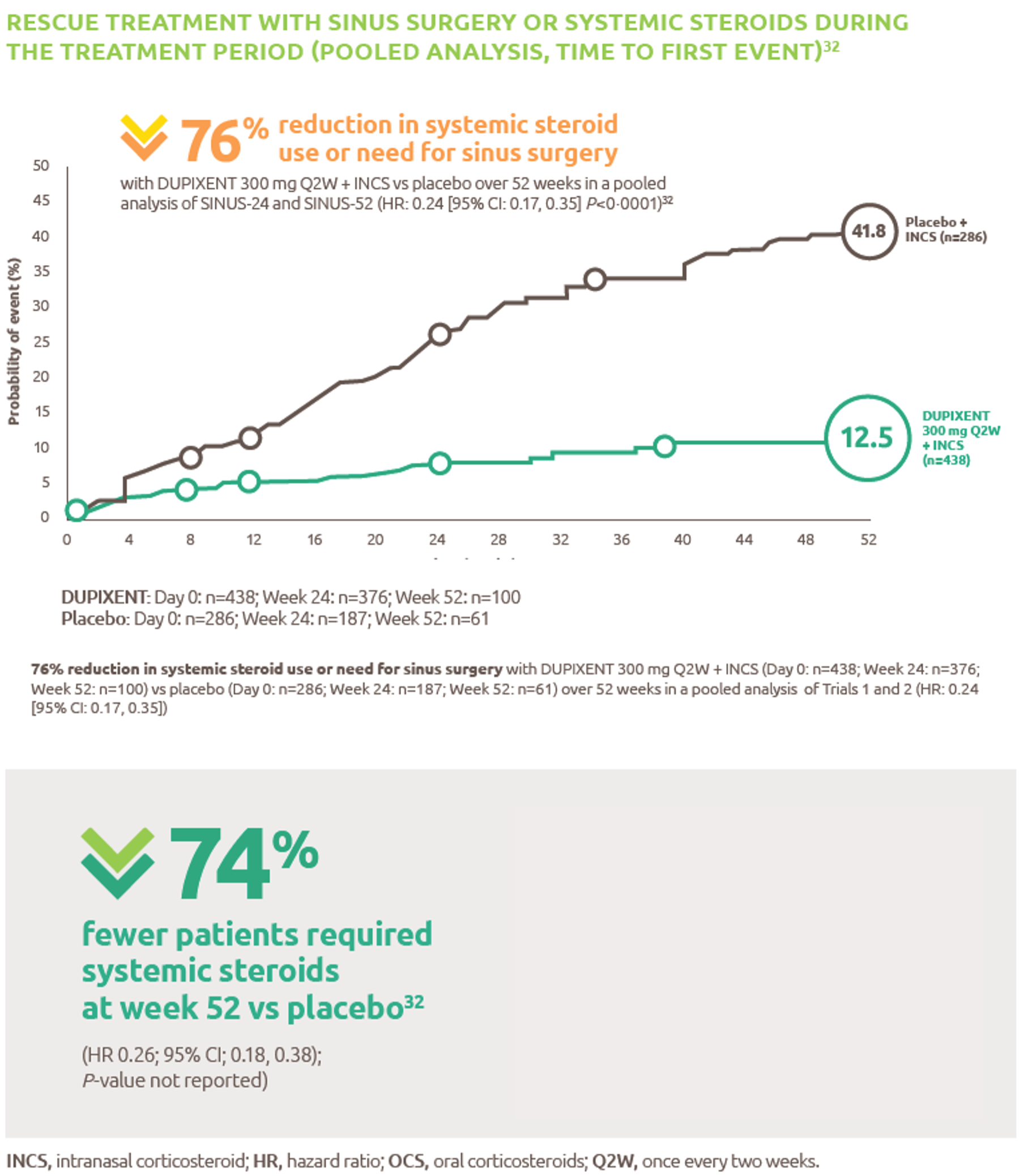
- Vaccination greatly reduces disease, disability, death and inequity worldwide. 2020. World Health Organization; [accessed 2020 Oct 2]. https://www.who.int/bulletin/volumes/86/2/07-040089/en/.
- Manufacturing vaccines is a complex journey. 2019. Sanofi; [accessed 2020 Oct 2]. https://www.sanofi.com/en/your-health/vaccines/production.
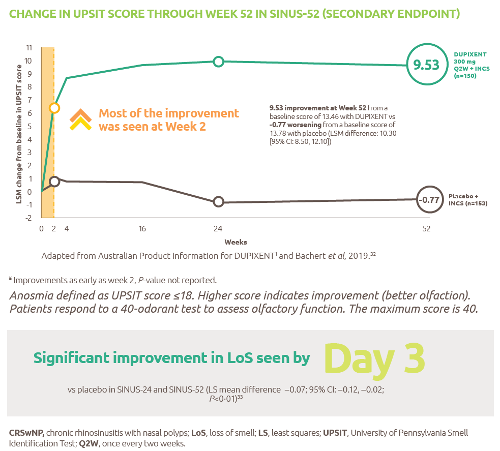
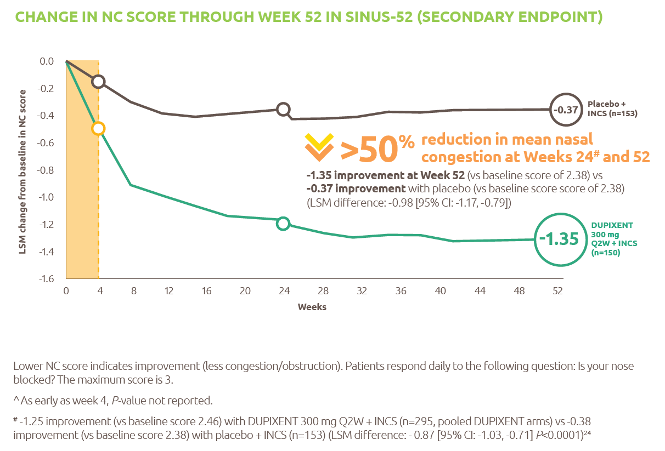
- The complex journey of a vaccine - part 1. 2016. IFPMA; [accessed 2020 Oct 2]. https://www.ifpma.org/wp-content/uploads/2014/01/IFPMA-ComplexJourney-Part_One_2016.pdf.
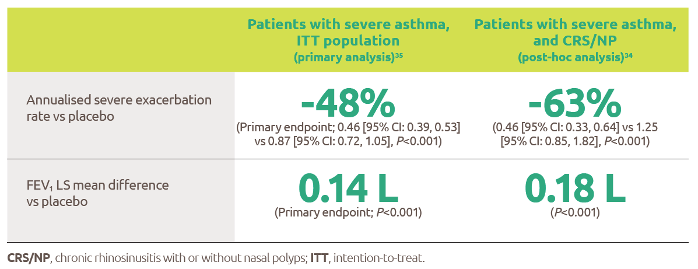
- Journey of vaccine: a complex manufacturing process. 2019. Sanofi; [accessed 2020 Oct 2]. https://www.sanofi.com/en/your-health/vaccines/production/journey-of-vaccine.
- Making vaccines. 2018. Sanofi; [accessed 2020 Oct 2]. https://www.youtube.com/watch?v=W-Zi5TitJzk.

.jpg/jcr:content/Hero_NNDSS%20(1).jpg)














.webp/jcr:content/RESP-ICT2-Wark_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Stone_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Tellus_400X300%20(1).webp)



.png)