Nexviazyme®
(avalglucosidase alfa)
Forced Vital Capacity (FVC)
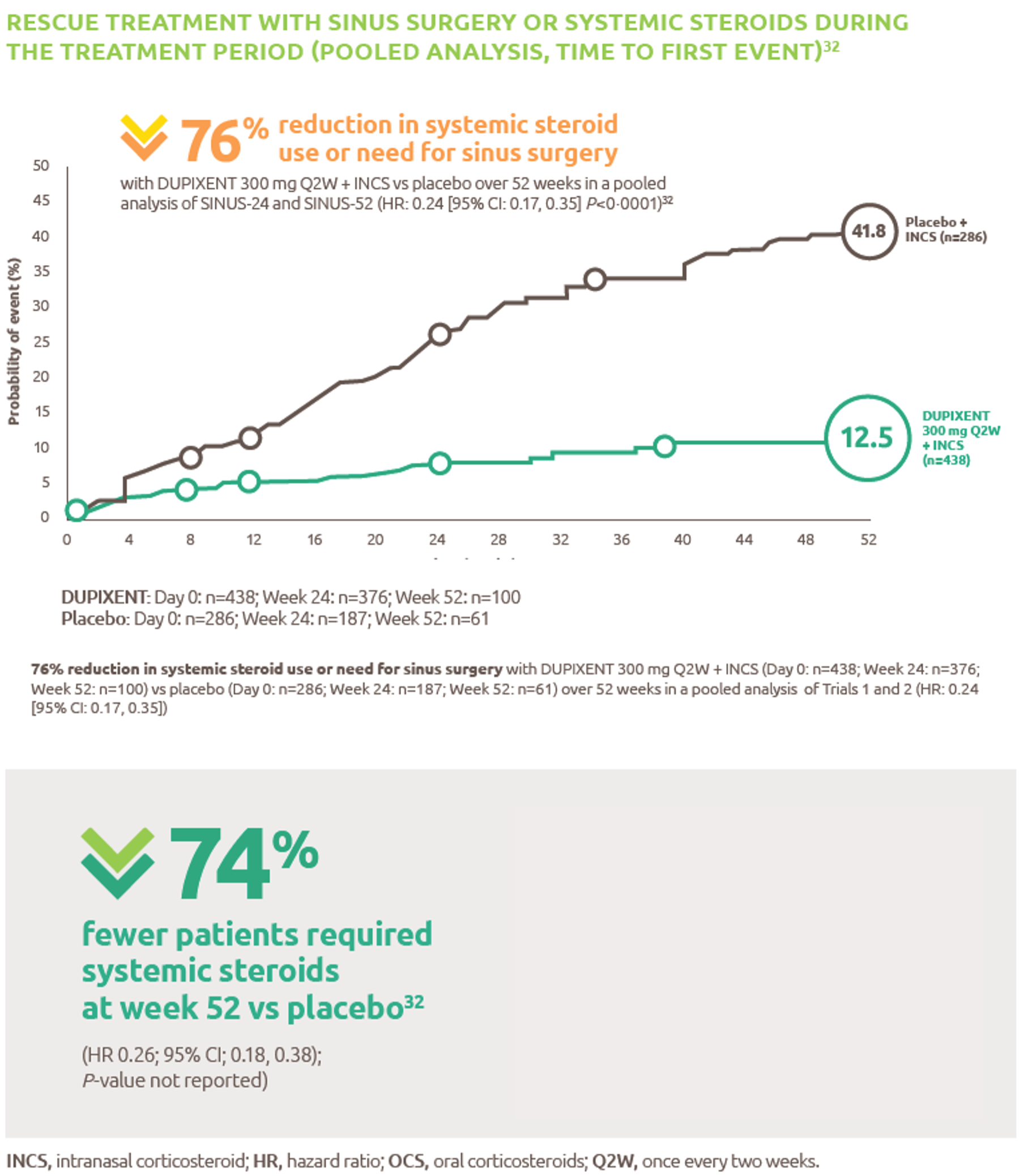
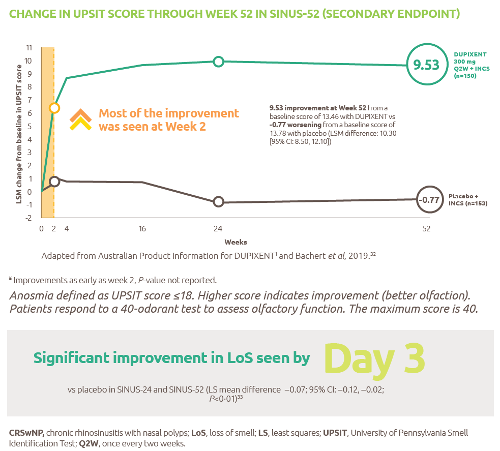
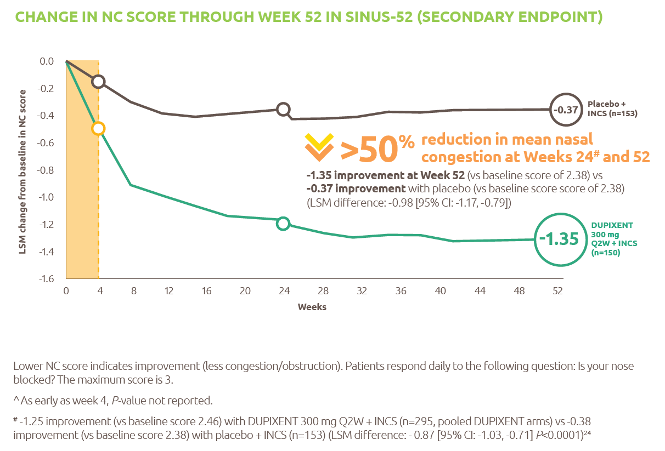
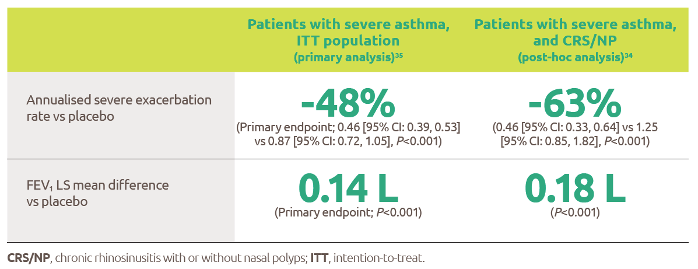
Nexviazyme has shown improved respiratory function in patients with LOPD vs Myozyme (P=0.06, not significant)1
Nexviazyme vs Myozyme: a 2.43% difference* in FVC (% predicted) (P=0.06, not significant).1
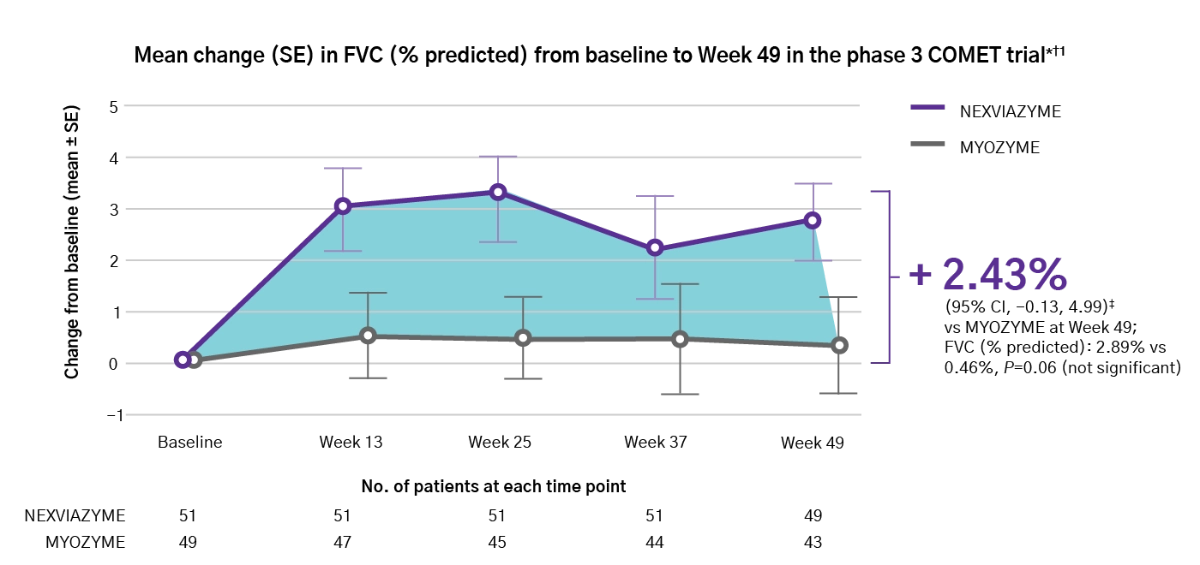
Study design: Patients were naive to treatment and aged 16 to 78 years at baseline. One hundred patients were randomised 1:1 to receive 20 mg/kg of Nexviazyme or Myozyme once every other week for 49 weeks.1 See Clinical trials for additional details.
*Mean (SD) pre-treatment baseline FVC (% predicted) values were 62.5 (14.4) and 61.6 (12.4) for the Nexviazyme and Myozyme treatment groups, respectively.1
†The difference in FVC (% predicted) exceeded the predefined noninferiority margin of -1.1 and achieved statistical noninferiority (P=0.0074).1
‡LS mean.1
CI, confidence interval; FVC, forced vital capacity; LOPD, late-onset Pompe disease; LS, least square; SD, standard deviation; SE, standard error.
- Nexviazyme Australian Approved Product Information.
PBS Information: NEXVIAZYME. This product is not listed on the PBS. This product is funded under the Life Saving Drugs Program.
Please review full Nexviazyme Product Information before prescribing. Full Product Information is available from sanofi-aventis australia pty ltd here or by contacting 1800 818 806.
▼ This medicinal product is subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse events at www.tga.gov.au/reporting-problems.
PBS Information: MYOZYME. This product is not listed on the PBS. This product is funded under the Life Saving Drugs Program.
Please review full Myozyme Product Information before prescribing. Full Product Information is available from sanofi-aventis australia pty ltd here or by contacting 1800 818 806.
Life Saving Drugs Program - Pompe disease - Guidelines are available at www.health.gov.au/resources/publications/lsdp-pompe-guidelines.
Nexviazyme® and Myozyme® are registered trademarks of Sanofi.
MAT-AU-2201155 - 2.0 - 05/2024
















.webp/jcr:content/RESP-ICT2-Wark_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Stone_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Tellus_400X300%20(1).webp)


.png)