Nexviazyme®
(avalglucosidase alfa)
Safety
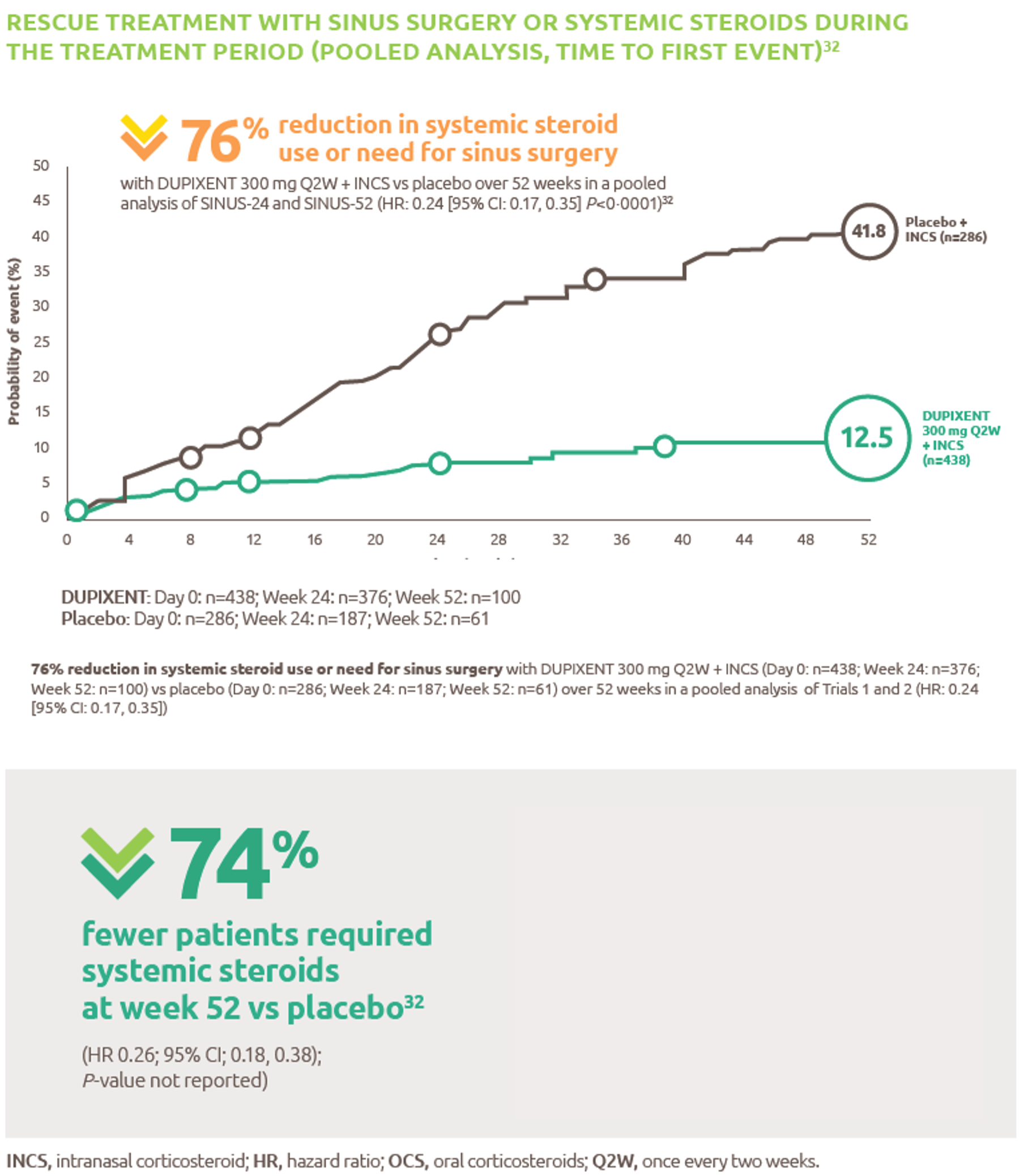
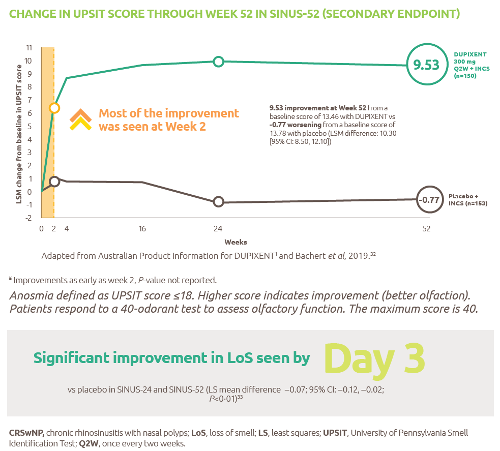
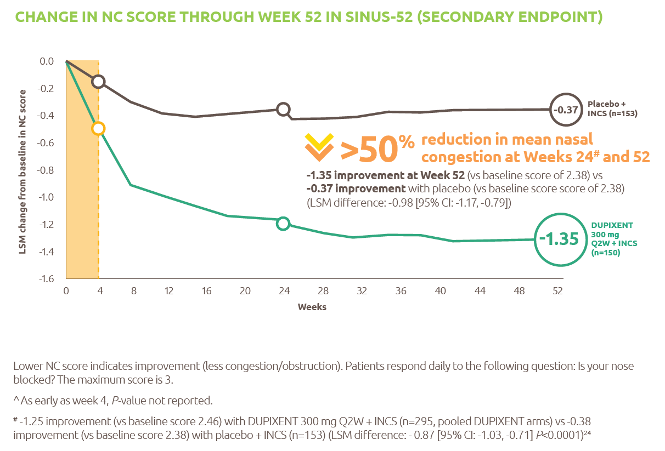
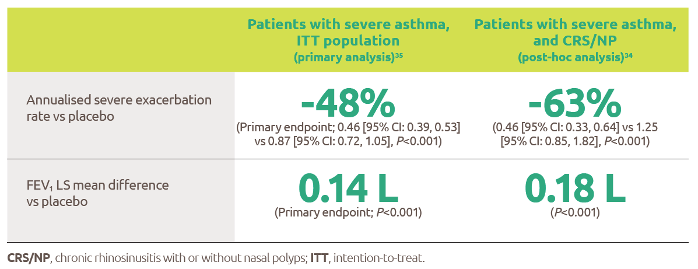
Nexviazyme: fewer patients with LOPD experienced IARs and serious adverse reactions* vs Myozyme (alglucosidase alfa)1,†
† P-value not assessed.
Safety profile of Nexviazyme in the phase 3 COMET trial

*Treatment-related.1
IARs1
Fewer patients experienced IARs with Nexviazyme (25.5%) compared with those receiving Myozyme (32.7%) (P-value not assessed).
Safety profile of Nexviazyme across 4 clinical trials1
A total of 138 patients (118 adults and 20 paediatric) were treated with Nexviazyme across 4 clinical trials. The most frequently reported adverse drug reactions (>5%) were pruritis, nausea, headache, rash, urticaria, chills, fatigue, and erythema.
In clinical studies, IARs were reported to occur in patients at any time during and/or within a few hours after the infusion of Nexviazyme and were more likely with higher infusion rates.
- 3 patients (2.2%) reported severe IARs including symptoms of chest discomfort, nausea, and increased blood pressure.
IARs were reported in 42 patients (30.4%). IARs reported in more than 1 patient included chills, cough, diarrhoea, erythema, fatigue, headache, influenza-like illness, nausea, ocular hyperaemia, pain in extremity, pruritus, rash, rash erythematous, tachycardia, urticaria, vomiting, chest discomfort, dizziness, hyperhidrosis, lip swelling, oxygen saturation decreased, pain, palmar erythema, swollen tongue and tremor. The majority of IARs were assessed as mild to moderate.
Discontinuations due to adverse reactions1
A total of 2 patients receiving NEXVIAZYME in clinical studies permanently discontinued treatment. Of these, 1 patient discontinued treatment because of a serious adverse event.
Immunogenicity1
- In adult patients, treatment-emergent ADAs were reported in both treatment-naive (95%) and treatment-experienced patients (49%). In the COMET trial, ADAs did not impact measures of efficacy while limited impacts on pharmacokinetics and pharmacodynamics were observed primarily with high-titre patients.
- In adult patients with LOPD, 1 treatment-naive patient and 1 treatment-experienced patient developed anaphylaxis.
- In paediatric patients with IOPD or LOPD, no patients developed anaphylactic reactions.
Nexviazyme has been evaluated for safety across the disease spectrum in children and adults1
Special populations
Paediatric and elderly populations
The safety of Nexviazyme was assessed in paediatric populations between 1 and 16 years of age (n=20) and in patients over the age of 75 years (n=3). In the paediatric population, Nexviazyme was studied in 19 patients with IOPD (1 to 12 years of age) and 1 patient with LOPD (16 years of age). In the elderly population, there is no recommended dose adjustment for patients over the age of 65.1
Patients with renal or hepatic impairment
Safety has not been studied in patients with hepatic impairment or patients with moderate or severe renal impairment. No dose adjustment is required in patients with mild renal impairment.1
Pharmacokinetic properties
Population pharmacokinetic analyses in patients with LOPD showed that age and gender did not meaningfully influence the pharmacokinetics of Nexviazyme.1
Special warnings and precautions for use
Hypersensitivity reactions including anaphylaxis
Hypersensitivity reactions, including anaphylaxis, have been reported in patients treated with Nexviazyme.1
- In clinical studies, 60 patients (43.5%) experienced hypersensitivity reactions, including 6 patients who reported severe hypersensitivity reactions and 2 patients who experienced anaphylaxis.1
- If mild or moderate hypersensitivity reactions occur, infusion rate may be slowed or temporarily ceased. If severe hypersensitivity or anaphylaxis occur, discontinue immediately and appropriate medical treatment should be initiated.1
Nexviazyme Product Information
For full safety information, refer to the Nexviazyme Product Information.
ADAs, antidrug antibodies; IARs, infusion associated reactions; IOPD, infantile-onset Pompe disease; LOPD, late-onset Pompe disease.
- Nexviazyme Australian Approved Product Information.
PBS Information: NEXVIAZYME. This product is not listed on the PBS. This product is funded under the Life Saving Drugs Program.
Please review full Nexviazyme Product Information before prescribing. Full Product Information is available from sanofi-aventis australia pty ltd here or by contacting 1800 818 806.
▼ This medicinal product is subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse events at www.tga.gov.au/reporting-problems.
PBS Information: MYOZYME. This product is not listed on the PBS. This product is funded under the Life Saving Drugs Program.
Please review full Myozyme Product Information before prescribing. Full Product Information is available from sanofi-aventis australia pty ltd here or by contacting 1800 818 806.
Life Saving Drugs Program - Pompe disease - Guidelines are available at www.health.gov.au/resources/publications/lsdp-pompe-guidelines.
Nexviazyme® and Myozyme® are registered trademarks of Sanofi.
MAT-AU-2201159 - 2.0 - 06/2024
















.webp/jcr:content/RESP-ICT2-Wark_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Stone_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Tellus_400X300%20(1).webp)


.png)