Nexviazyme®
(avalglucosidase alfa)
Muscle Strength
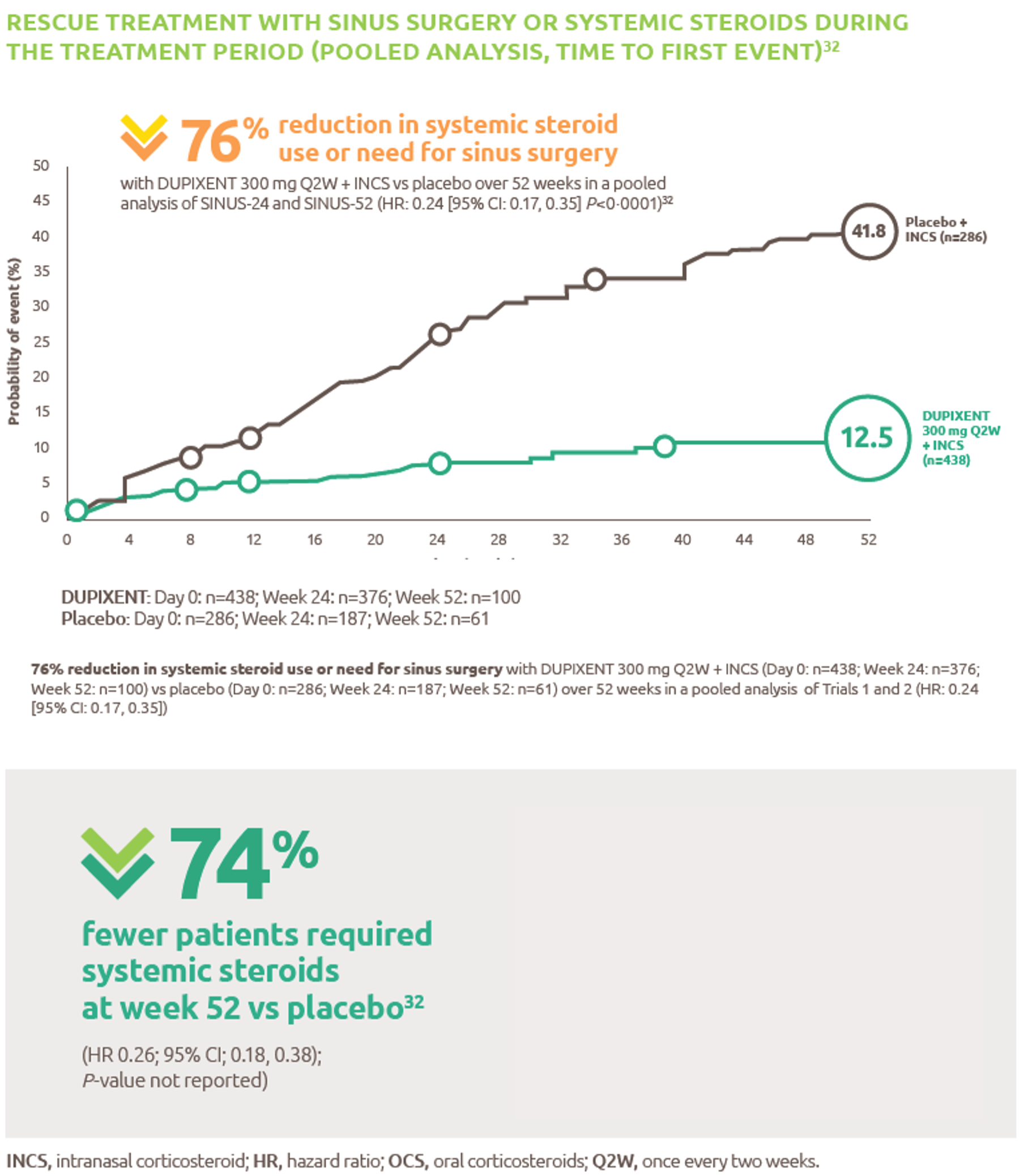
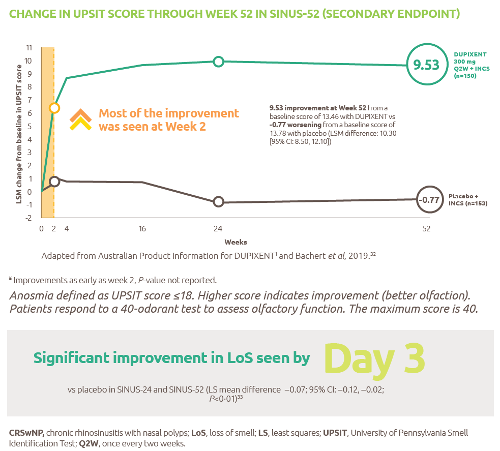
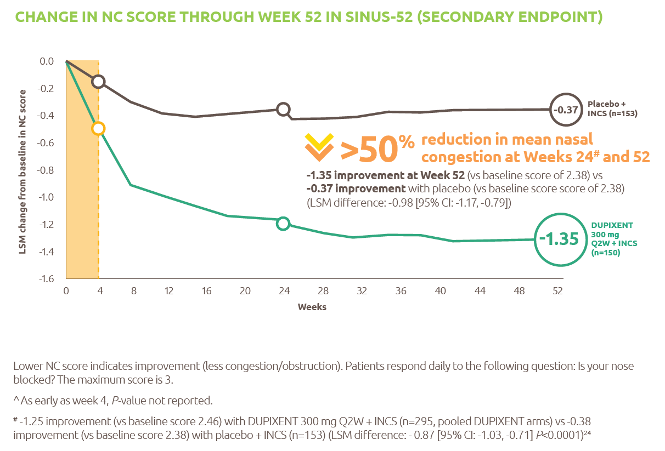
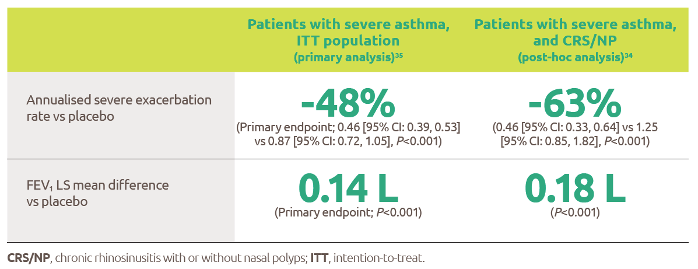
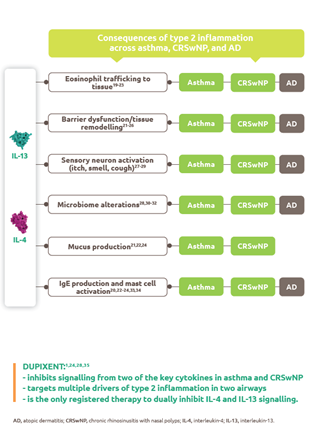
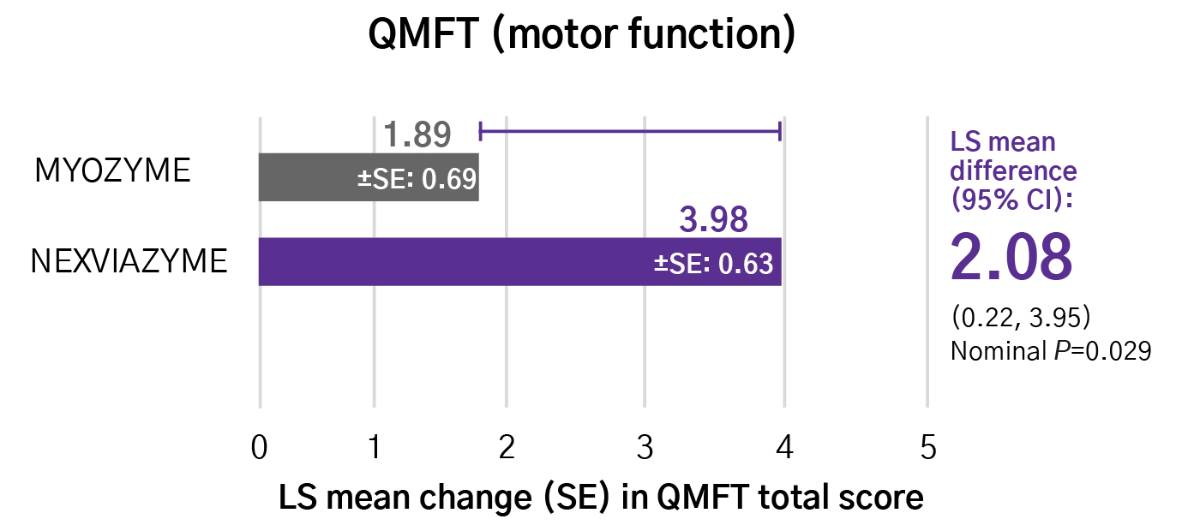
Help to improve muscle strength and motor function with Nexviazyme1,2
Greater numerical improvements were observed across all key measures of muscle strength and motor function, including HHD, QMFT, MIP, and MEP in patients treated with Nexviazyme vs Myozyme (alglucosidase alfa) at Week 49 (nominal P-value = not significant)*1,2
Muscle strength and function†
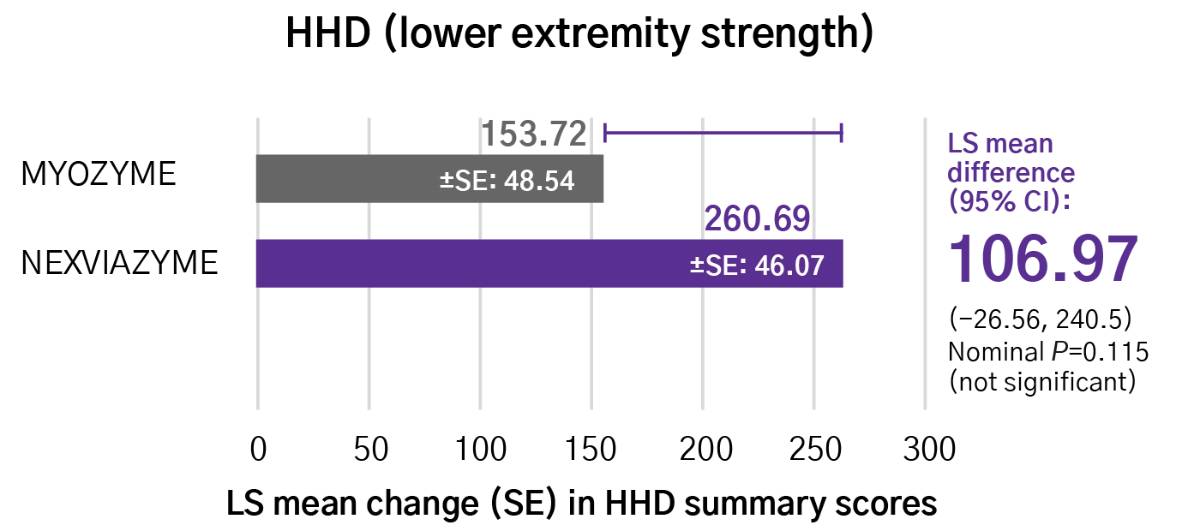
Respiratory muscle strength†‡
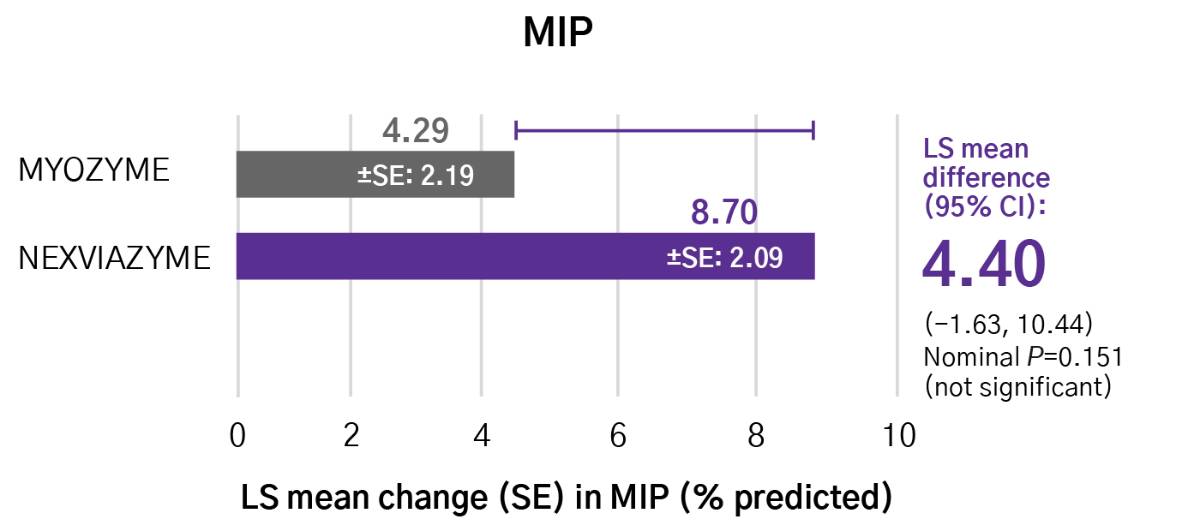
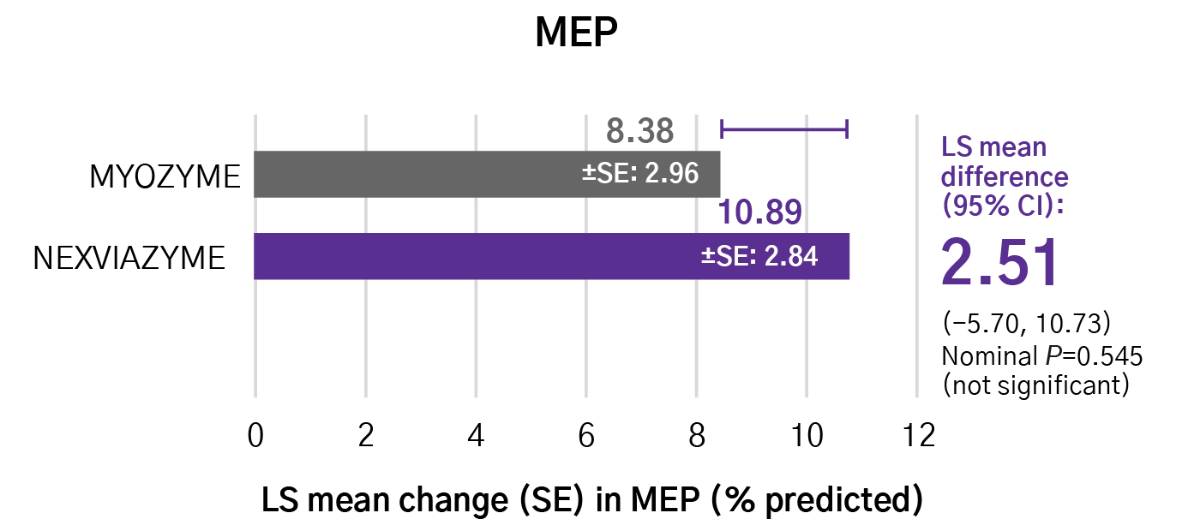
*Mixed model for repeated measures was used to test primary and secondary endpoints. As superiority was not established for FVC% predicted (P=0.06), formal testing was stopped as per the protocol hierarchy; P-values generated for secondary endpoints are at nominal level without multiplicity adjustment.1,2
†Testing was performed without multiplicity adjustment.1
‡Four participants (two in each group) with implausibly high MIP% predicted and MEP% predicted values at baseline were excluded from all MIP and MEP analyses.1,2
CI, confidence interval; HHD, handheld dynamometry; LS, least square; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; QMFT, quick motor function test; SE, standard error.
- Nexviazyme Australian Approved Product Information.
- DIaz-Manera et al Lancet Neurol 2021; 20: 1012–26.
PBS Information: NEXVIAZYME. This product is not listed on the PBS. This product is funded under the Life Saving Drugs Program.
Please review full Nexviazyme Product Information before prescribing. Full Product Information is available from sanofi-aventis australia pty ltd here or by contacting 1800 818 806.
▼ This medicinal product is subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse events at www.tga.gov.au/reporting-problems.
PBS Information: MYOZYME. This product is not listed on the PBS. This product is funded under the Life Saving Drugs Program.
Please review full Myozyme Product Information before prescribing. Full Product Information is available from sanofi-aventis australia pty ltd here or by contacting 1800 818 806.
Life Saving Drugs Program - Pompe disease - Guidelines are available at www.health.gov.au/resources/publications/lsdp-pompe-guidelines.
Nexviazyme® and Myozyme® are registered trademarks of Sanofi.
MAT-AU-2201157 - 2.0 - 05/2024
















.webp/jcr:content/RESP-ICT2-Wark_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Stone_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Tellus_400X300%20(1).webp)


.png)