Quest, Venture, and Traverse (DRI12544) Clinical Studies
.jpg0/jcr:content/Hero_Atopic%20Derm_2120x862px_v0.2%20(1).jpg)
The asthma development program included three randomised, double-blind, placebo-controlled, parallel-group, multi-centre studies (DRI12544, Quest, and Venture) of 24 to 52 weeks in treatment duration which enrolled a total of 2888 patients (12 years of age and older).1
Of these, 2678 had a history of 1 or more severe exacerbations in the year prior to enrolment despite regular use of medium to high-dose inhaled corticosteroids plus an additional controller(s) (DRI12544 and Quest).1
A total of 210 patients with oral corticosteroid-dependent asthma receiving high-dose inhaled corticosteroids plus up to two additional controllers were enrolled (Venture).1
Quest Study Design
Quest was a phase 3, randomised, double-blind, placebo controlled, parallel-group study in patients aged ≥12 years with uncontrolled moderate-to-severe asthma.1
Quest included 1902 patients (12 years of age and older). Dupixent (dupilumab) compared with placebo was evaluated in 107 adolescent and 1795 adult patients with moderate-to-severe asthma on a medium- or high- dose inhaled corticosteroid (ICS) and a minimum of one and up to two controller medications.
Patients requiring a third controller were allowed to participate in this study. Patients were randomised to receive either 200 mg (n=631) or 300 mg (n=633) Dupixent every other week (or matching placebo for either 200 mg [n=317] or 300 mg [n= 321] every other week) following an initial dose of 400 mg, 600 mg or placebo respectively.1
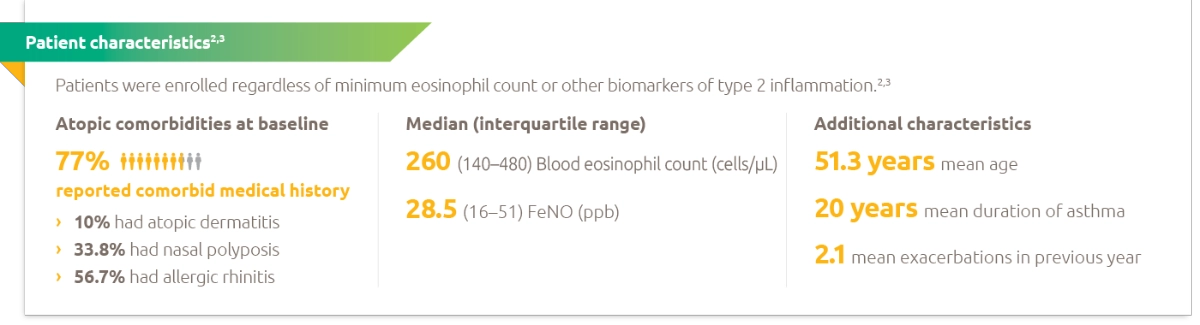
The primary endpoints were the annualised rate of severe exacerbation events during the 52-week placebo controlled period and change from baseline in pre-bronchodilator FEV1 at Week 12 in overall population (unrestricted by minimum baseline eosinophils or other Type 2 biomarkers).1
Additional secondary endpoints included exacerbation rates and FEV1 in patients with different baseline levels of eosinophils as well as mean change from baseline and responder rates in the ACQ-5 and AQLQ(S) scores.1

*With 400 mg loading dose.
†With 600 mg loading dose.
A severe exacerbation event was defined as a deterioration of asthma requiring use of systemic corticosteriods for ≥3 days or hospitalisation or emergency department visit leading to treatment with systemic corticosteroids.1
The population included patients on medium-to-high dose ICS (fluticasone propionate ≥500 µg/day or equipotent equivalent). Up to 2 additional controllers (e.g. LABA or LTRA) were allowed.1
Venture Study Design
Venture was a phase 3, randomised, double-blind, placebo controlled study in patients aged ≥12 years with oral glucocorticoid-dependent severe asthma.3
Venture was a 24-week oral corticosteroid-reduction study in 210 patients with asthma who required daily oral corticosteroids in addition to regular use of high dose inhaled corticosteroids plus an additional controller.
After optimising the OCS dose during the screening period, patients received 300 mg Dupixent (n=103) or placebo (n=107) once every other week for 24 weeks following an initial dose of 600 mg or placebo.3
Patients continued to receive their existing asthma medicine during the study; however, their OCS dose which was reduced every 4 weeks during the OCS reduction phase (Week 4-20), as long as asthma control was maintained. The OCS reduction was performed according to algorithm specified in the protocol.3
The primary endpoint was the percent reduction of oral corticosteroid dose at Week 24 compared with the baseline dose, while maintaining asthma control in the overall population (unrestricted by minimum baseline eosinophils or other Type 2 biomarkers). The key secondary endpoints were the proportion of patients achieving a reduction of 50% or greater in their OCS dose compared with baseline and proportion of patients achieving a reduction of OCS dose to <5 mg/day at Week 24 while maintaining asthma control.3
Additional secondary endpoints included the annualised rate of severe exacerbation events during treatment period and mean change from baseline and responder rate in the ACQ-5 and AQLQ(S) scores.3

*With 600 mg loading dose.
†OCS dose was reduced every 4 weeks during the OCS reduction phase (week 4-20), as long as asthma control was maintained.
OCS-dependent patients required daily OCS in the previous 6 months (5-35 mg/day of prednisone or prednisolone or equivalent) in addition to regular use of high-dose ICS (fluticasone propionate ≥500 µg/day or equipotent equivalent) plus up to 2 additional controllers (e.g. LABA or LTRA).
Asthma control was defined as no clinically significant event (investigator's judgement) leading to an upward adjustment in the oral glucocorticoid dose between weeks 20 and 24 (maintenance phase).
Traverse Study Design
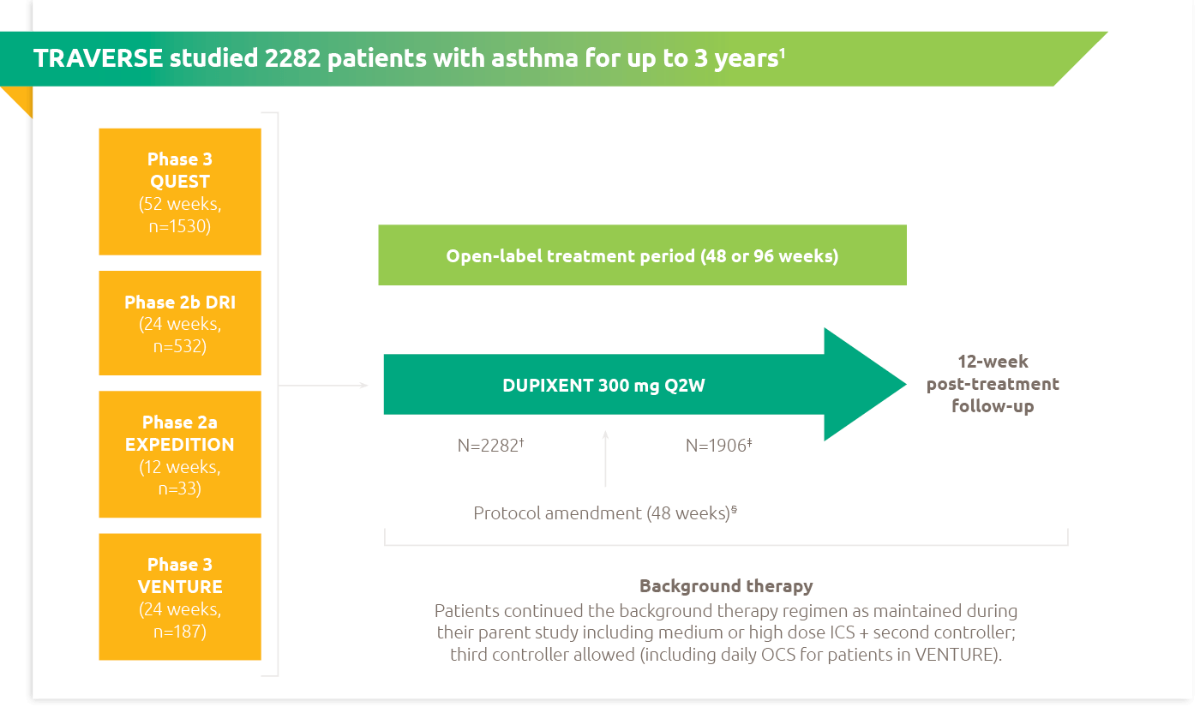
Traverse was an open-label extension study of 2282 patients (adults and adolescents) with moderate-to-severe or oral-corticosteroid-dependent severe asthma who had completed a previous Dupixent asthma study.4
The primary endpoint was the number and percentage of patients with any treatment-emergent adverse events.
Secondary endpoints included annualised exacerbation rate over the treatment period (up to week 96), change from baseline in pre-bronchodilator FEV1 (up to week 96), Type 2 biomarker levels, such as blood eosinophils and serum total IgE (up to week 96)*, anti-drug antibodies (up to week 96), and ACQ-5 and AQLQ (up to week 48)4
*Serum total IgE was assessed in patients from the phase 2b study (DRI) only.
No p-values were assessed and only observed data was used as TRAVERSE was an open-label, single-group study with no comparator group, and as patients were enrolled from four parent studies with varying duration, dose exposures, enrolment criteria, and background therapies. Efficacy endpoints are presented as change from parent study baseline over the treatment period.4
†Two patients from the VENTURE study were enrolled in error and were prematurely discontinued from the study without exposure to DUPIXENT. Total number of patients enrolled and exposed to treatment in the OLE.4
‡Total number of patients who continued to be exposed to treatment beyond 48 weeks.4
§Amendment of treatment period was made from 96 weeks to 48 weeks due to accumulating safety data for DUPIXENT across multiple indications in a clinical trial setting.4
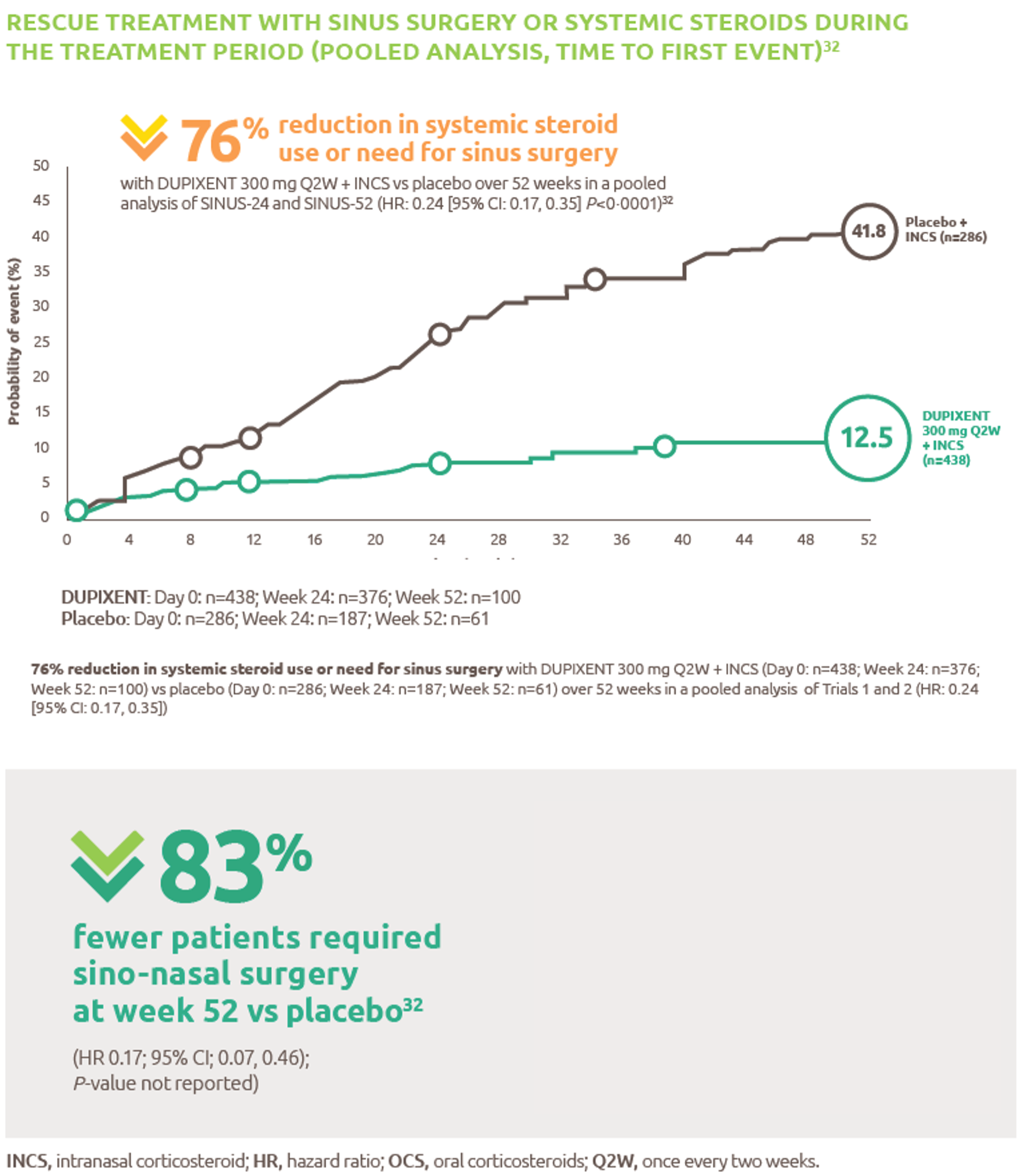
Annualised exacerbation rate was defined as severe asthma exacerbations requiring systemic corticosteroids for ≥3 days, hospitalisation, or emergency room visit.
ACQ-5, five-item asthma control questionnaire; AQLQ, asthma quality of life questionnaire; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid; IgE, immunoglobulin E; LABA, long-acting beta agonist; LTRA, leukotriene-receptior antagonist; OCS, oral corticosteroid; OLE, open-label extension; Q2W, every 2 weeks; ppb, parts per billion SOC, standard of care.
- Castro M et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Eng J Med 2018; 378(26): 2486-96 (and supplementary appendix).
- Australian Approved Product Information for DUPIXENT (dupilumab).
- Rabe KF et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378(26): 2475–85 (and supplementary appendix).
- Wechsler ME et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med 2022; 10(1): 11-25(and supplementary appendix).















.webp/jcr:content/RESP-ICT2-Wark_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Stone_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Tellus_400X300%20(1).webp)



.png)